+ LoC
(Periodic Table & Atoms)
Which of the following is correct about particles in a liquid?
A) They are closer together and lower in energy than those in a solid.
B) They are closer together and lower in energy than those in a gas.
C) They are farther apart and higher in energy than those in a gas.
D) They are farther apart and lower in energy than those in a solid.
B
What type of change or property can be observed without altering the identity of a substance?
Physical Change/Physical Property
A substance that has a pH above 7 would be classified as a(n)?
Base
Which of the following are compounds?
carbon, gold, oxygen, water, nitrogen
water
John discovered that sodium (Na) is found in Group 1 on the Periodic Table. Where should John look for another element that has similar properties?
in the same column
Which of the following examples demonstrates replication?
A) Joseph conducts his own experiment 25 times.
B) Sarah is following another student’s written procedures for an experiment that was already performed.
C) Juanita conducts her experiment 1 time.
D) Matt writes the procedures of his experiment carefully and carries out his experiment.
B
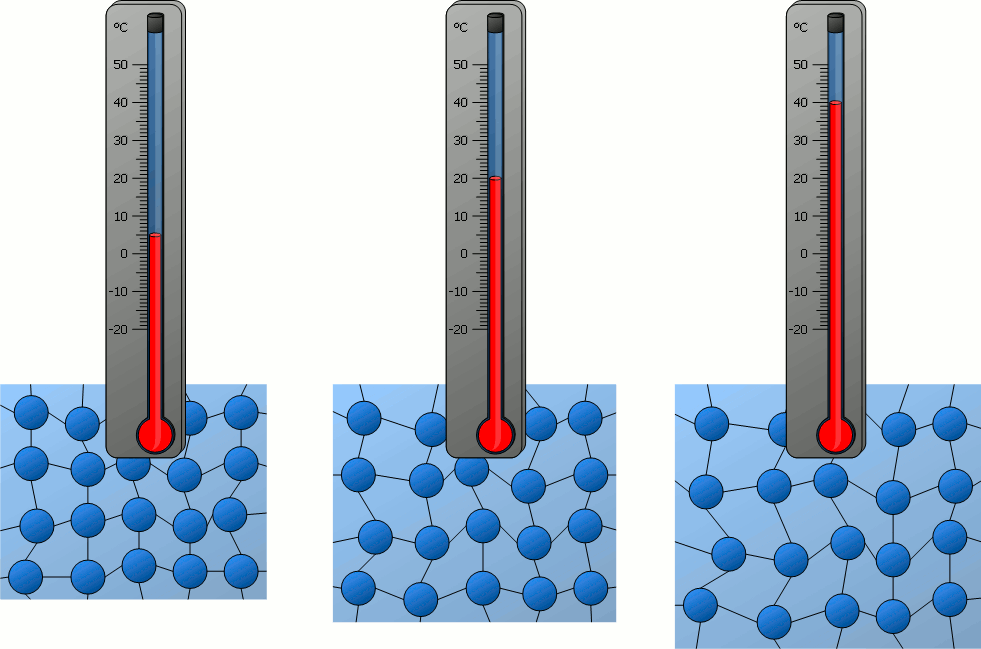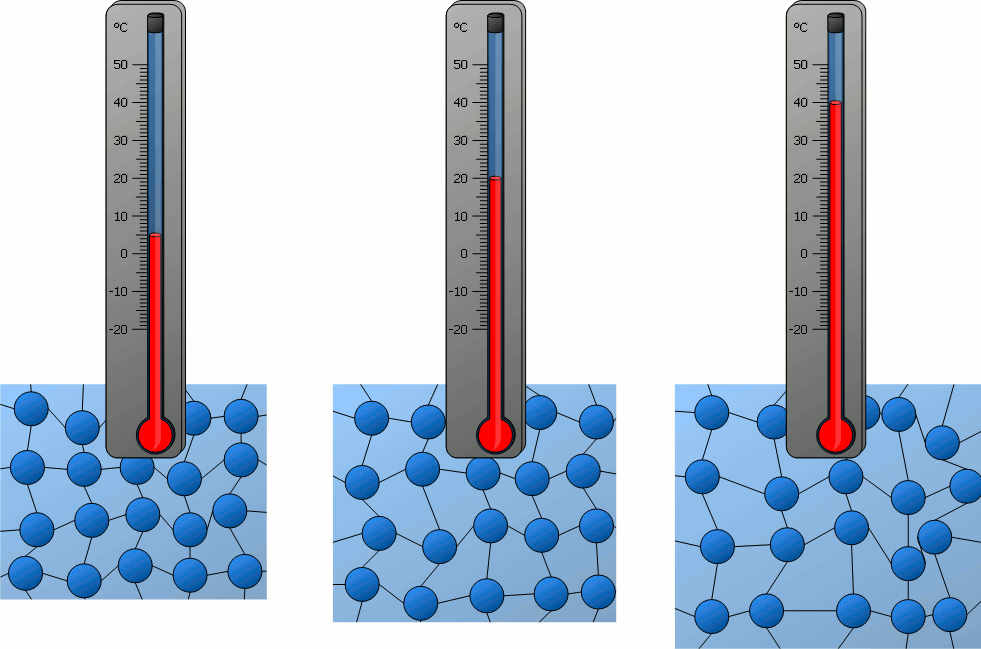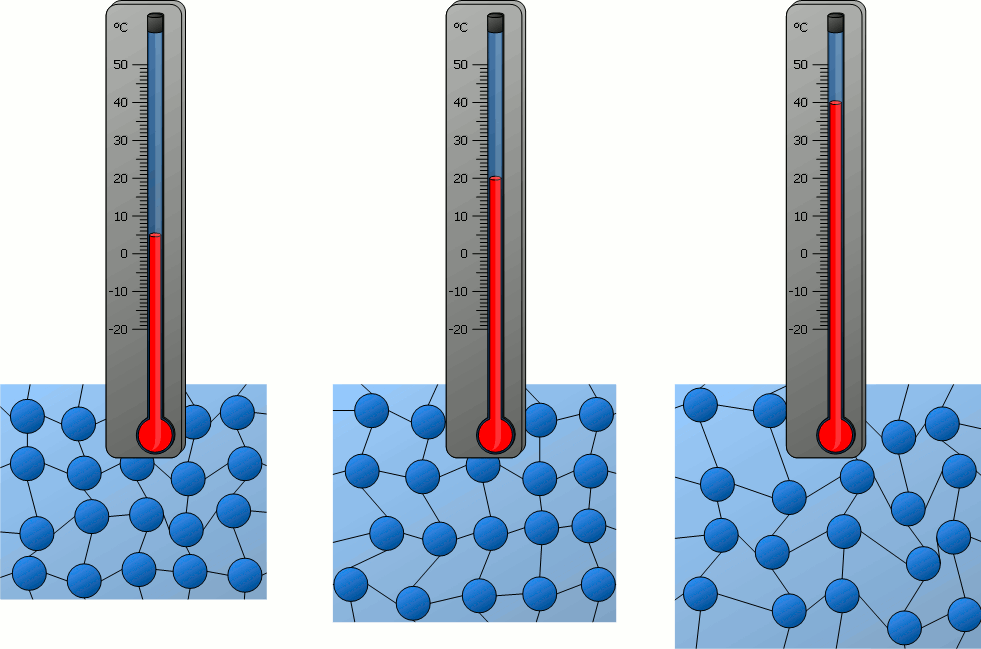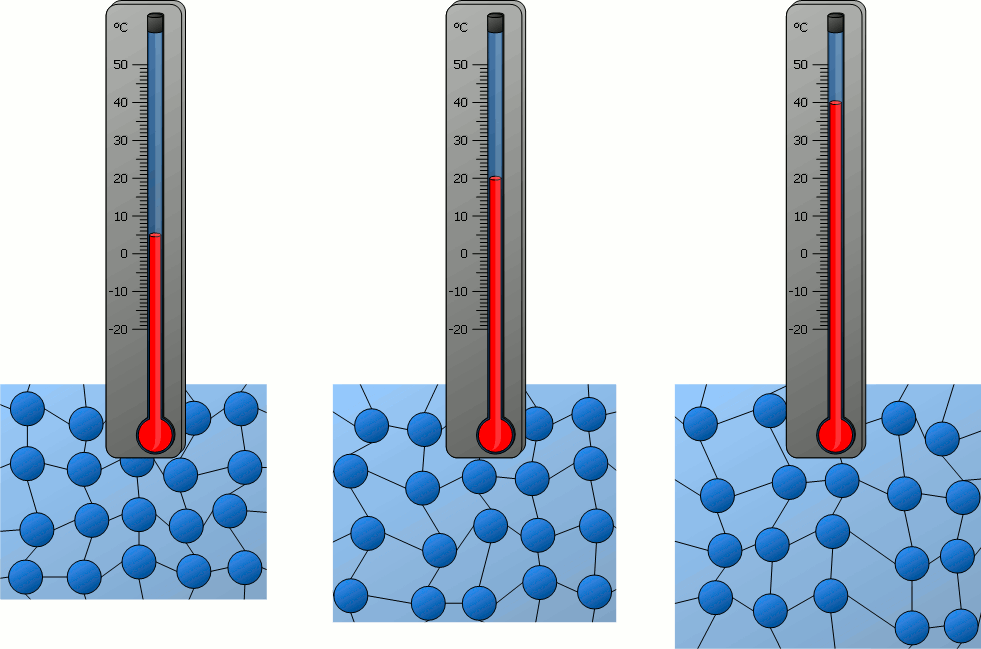
How do the particles in an object at 40°C compare to the particles of an object at 0ºC?
A) The particles at 40°C are moving faster than the particles at 0°C.
B) The particles at 0°C are moving faster than the particles at 40°C.
C) The particles at 40°C are moving at the same speed as the particles at 0°C.
D) Neither the particles at 40°C or the particles at 0°C are moving.
A
The ability to conduct an electric current, dissolve in water and melt are all examples of...
A) chemical bonding B) chemical properties
C) physical changes D) physical properties
D
The label on a chemical in a science laboratory reads “Strong Acid.” Based on this label, what can a student best determine about the chemical?
A) It is a clear liquid B) It has a high density
C) It has a low pH D) It has a low melting point
C
Which figure best illustrates a pure substance and why?
Answers may vary. Accept X, Y, and/or Z provided that reasoning refers to X being an element, Y and Z being compounds and that elements and compounds are pure substances.
Which of the following pairs of elements have properties that are the LEAST similar?
A) He and Xe B) Cl and I C) N and P D) Si and S
D) Si and S
Which of the following shows repeated trials in an experiment but does NOT show replication?
A) Samuel writes his own procedures and then has a friend complete 20 trials.
B) Leslie writes her own procedures for her group to follow.
C) Joshua conducts an experiment by following the procedures given to him by his teacher.
D) Katelyn conducts an experiment that requires her to throw an object at a target 20 times.
D
What is one conclusion you can make about particles in solids, liquids and gasses based on the beakers in the image above?
Answers will vary. Accept all reasonable answers related to temperature, density, speed of movement
Why does increasing the temperature of a chemical reaction increase the reaction rate?
Accept all answers similar to "Faster moving particles come in contact more often, which gives more chances for a reaction to happen."
Melanie is given an unknown substance by her teacher. Melanie's teacher tells her that the substance has a bitter taste. Melanie also observes that the substance is slippery. These properties indicate that the substance may be a(n)...
A) Acid B) Base C) Salt D) water
B) Base
Tia is making a drink using Kool Aid powder and water. Identify the solvent and solute in this scenario.
Kool Aid=solute Water=solvent
Which subatomic particle is the arrow pointing to in the diagram below?
proton
Rashawn completes 30 trials in his experiment and has 2 of his friends complete the experiment by following his procedures. Which part of the scenario above demonstrates repetition?
30 trials
A B C
Give an example for the state of matter shown in each picture.
Answers will vary, but accept all answers in alignment with A) solid B) liquid C) gas.
All examples must be correct for points to be awarded.
What would the missing product from the reaction below consist of?
One oxygen atom and three hydrogen atoms
The density of water is 1 g/cm3. Explain why an object with a density of 0.5 g/cm3 will float in water.
Answers may vary. Accept all reasonable & accurate answers related to objects with density less than density of water will float, or discussing the space between particles of the object being less than the space between water particles.
Draw or List an example of a heterogeneous mixture. What information can you provide to support what you drew/wrote?
Answers will vary. Accept all reasonable answers showing/explaining components not being chemically combined, being unevenly mixed, etc.
What color are the electrons in the diagram below?
yellow
How is replication different from repetition?
Replication is done by others. Replication is done by the original experimenters.
Draw a model that shows how particles move before AND after being heated.
A substance (X) underwent a chemical change to form two new substances (Y and Z). Use the numbers "1", "2" and "3" to write an equation that models this situation AND illustrates the Law of Conservation of Mass.
3 = 2 + 1
According to the Law of Conservation of Mass, what will be the mass of the product in a closed system if 300 grams of hydrogen peroxide are combined with 200 grams of manganese dioxide in a chemical reaction?
A) 500 grams B) 300 grams C) 200 grams D) 100 grams
A) 500 grams (300+200=500)
"Mixtures may contain compounds, but compounds cannot contain mixtures." What facts or examples could you use to explain this statement?
Answers will vary. Accept all reasonable answers related to the parts of compounds being chemically joined vs. the parts of solutions/mixtures which are NOT chemically joined.
Based on the Periodic Table... What facts would you select to support the idea that Beryllium (Be) has less atomic mass than Iron (Fe)?
Answers will vary. Accept all reasonable answers related to Iron's greater atomic number, number of protons and/or neutrons.
How are repetition and replication useful to scientists?
Both processes confirm results.