Light has the properties of these two things.
What are particles and waves?
E = hv
What is the energy of a photon
The probability of finding the particle within the first two peaks for the n=3 state.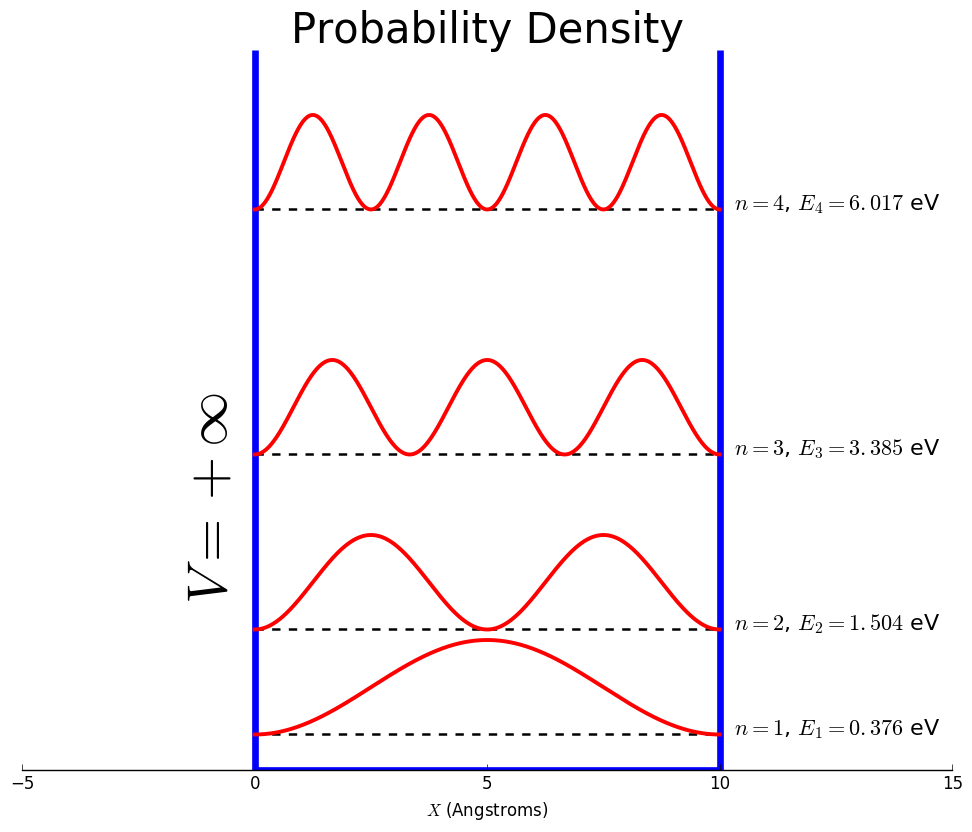
What is 2/3?
The allowed quantum number values for particle in a box.
What is n=1, 2, 3, ...?
Two electrons in the same orbital must have this important property.
What is anti-symmetric spins?
This person had the idea that particles have wave-particle duality?
Who is De Broglie?
E= -13.60*(Z2)/(n2)
What is the energy for a hydrogen atom?
The De Broglie wavelength for an electron moving at half the speed of light.
What is 4.85x10-12 m?
The model for the shown wavefunctions.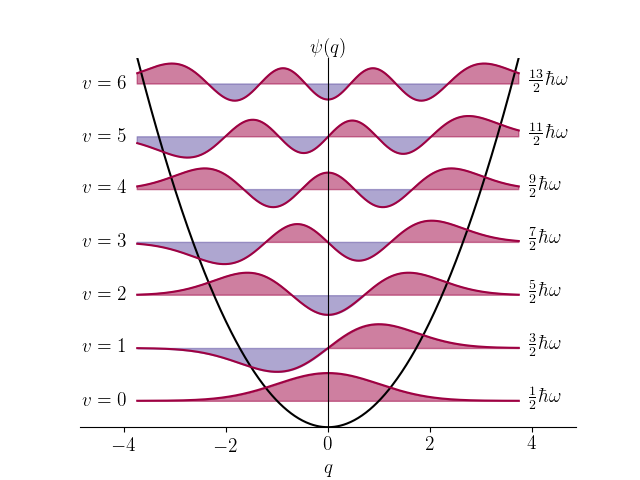
What is the harmonic oscillator model?
The conditions for the formation of molecular orbitals.
What is symmetry and similar energy?
The major conclusion of Planck from the Blackbody radiation problem.
What is the quantization of energy?
E = (v+1/2)h
What is the energy equation for a harmonic oscillator?
The Reduced mass for 16O19F molecule.
What is 1.44x10-26 kg?
This model's wavefunctions look very similar to the many atomic orbitals we have seen.
What is the rigid rotor?
The probability of finding a particle at a node in a wave-function.
What is zero?
The definition of the Heisenberg Uncertainty Principle.
What is being unable to know simultaneously both the position and momentum of a particle?
v = (1/2π)√(k/μ)
What is the formula for the harmonic frequency?
The ionization energy for a Be3+ atom.
What is -217.6 eV?
What is the electron-electron repulsion piece?
The term for when multiple states have the same energy.
What is degenerate?
The three conditions for a well behaved wave-function.
What is single valued, continuous, and quadratically integrable?
ΔE= 2Beh(J+1)
What is the energy for a rotational transition?
The energy of the 2nd vibrational excited state for a molecule with a fundamental frequency of 5.00x1013 Hz.
What is 8.28x10-20 J.
The wave function for the n=3 state for a particle in a box.
What is ψ3= √(2/l)sin(3πx/l)
How do we get hybrid orbitals using MO theory.
What is linear combinations of molecular orbitals?
