what elements atomic number is 26
iron
the small, positively charged center of the atom
the nucleus
the smallest piece of matter that still has the properties of the element
an atom

what element is this
carbon
an arrangement of the elements by increasing atomic number and by changes in physical and chemical properties.
periodic table
particle with an electrical charge of 1-
electron
whats the definition of an element
a basic part of a whole
a number equal to the number of protons in an atom
atomic number
What is the atomic number

2
Na on the periodic table
sodium
particle with no charge
neutron
how does static electricity work
the result of an imbalance between negative and positive charges in an object
What is the atomic weight

107.87
what elements atomic number is 27
cobalt
These two particles make up the net charge of an atom
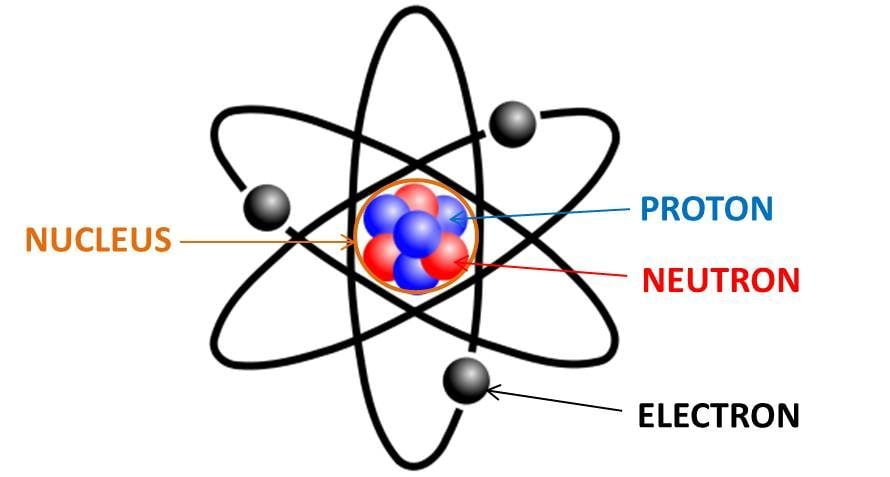
protons and electrons
particle with an electrical charge of 1+
proton
What is a molecule, and how would you recognize one
A molecule is two or more atoms connected by chemical bonds, which form the smallest unit of a substance that retains the composition and properties of that substance.
what is a molecule
a group of two or more atoms that form the smallest identifiable unit
This particle is shown on the rings of the Bohr diagram

electrons
if the atomic number is 6, how many protons does it have?

6
the area around the nucleus of an atom where its electrons are most likely found
electron cloud
if the atomic number is 11, how many electrons does it have
11
the sum of the number of protons and the number of neutrons in an atom
the mass number
how does static electricity work
Static electricity is the result of an imbalance between negative and positive charges in an object.
what elements number is 71
lutetium