What is an isotope?
Same number of protons, different number of neutrons.
Which decay results in a helium nucleus being released?
What is Alpha decay
What is the following model of an atom called?
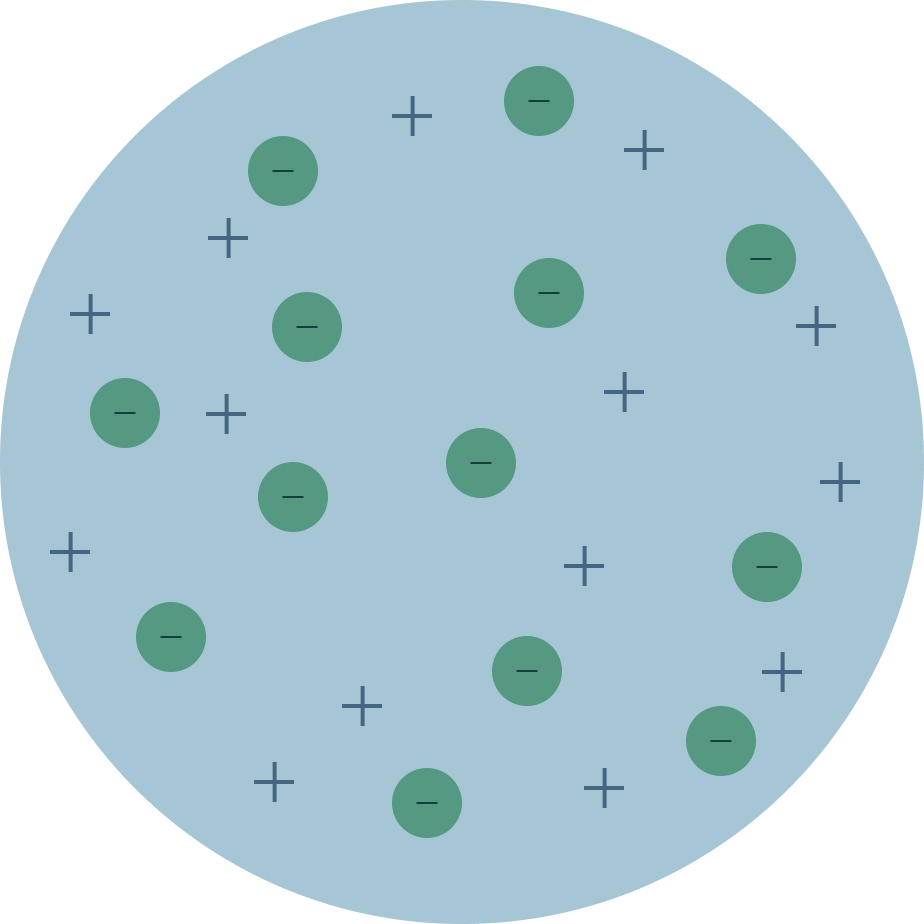
Plum Pudding Model
If a Fluorine atom has 9 protons, why can we assume it also has 9 electrons?
Atoms are electronically neutral
The element actinium has an atomic number of 89 and a mass of 227. What will the new element be if it went through a beta minus decay?
Thorium
Type of radiation that removes electrons from atoms
What is ionising radiation
What do we use to measure radiation?
Geiger Muller Counter
How many neutrons does Rh-103 have?
58 neutrons
Berkelium has an atomic number of 97. What will the new atomic number be if the element goes through a alpha decay?
What is 95
Which of the following shows an oxygen atom that has gained 2 electrons?
O2+ O O2-
O2-
3115P --> 0+1e + ???
3116S
Why does Chlorine have an atomic mass of 35.5 on the periodic table?
Mass on the table is an average of all the isotopes of that atom.
??? --> 42He + 59 28Ni
6330Zn