Isotopes
Radioactive Decay
Half-Life
Nuclear Energy
WILD!
100
Isotopes of a given element will have a different number of this particle
What is different number of neutrons
100
What causes radioactive decay?
What is a radioisotope with an unstable nucleus.
100
At two half-lives, what proportion of parent nuclei remain?
What is 1/4, or 25%
100
Explain at least two differences between nuclear fission and nuclear fusion
What are: any two of the following:


100
Name 2 elements that Marie & Pierre Curie discovered.
What are thorium, radium, polonium, and uranium
200
What is the other half of the common isotope pair that includes Rubidium-87?
What is strontium-87 (on your data package)
200
Rank the types of radioactive decay from strongest to weakest
What is gamma, beta, alpha
200
The half-life of an isotope is 12,000 years. If 87.5% of a sample of this isotope contains daughter isotope, how old is the sample?
What is 36,000 years old.
200
What is artificial radioactivity?
What is the creation of radioisotopes by firing particles at the nucleus of a stable isotope to induce radioactivity.
200
Do atoms decay at a constant rate?
What is no, decay rate decreases over time as the number of parent atoms decreases (rate = # atoms decayed/time)
300
Krypton-80 has this many neutrons
What is 44
300
Identify the type of decay in the equation below
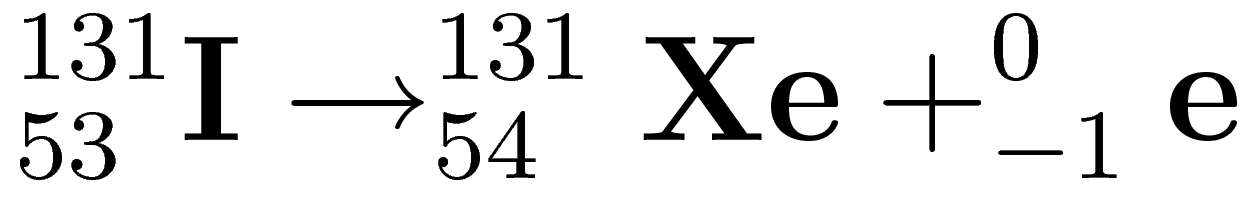
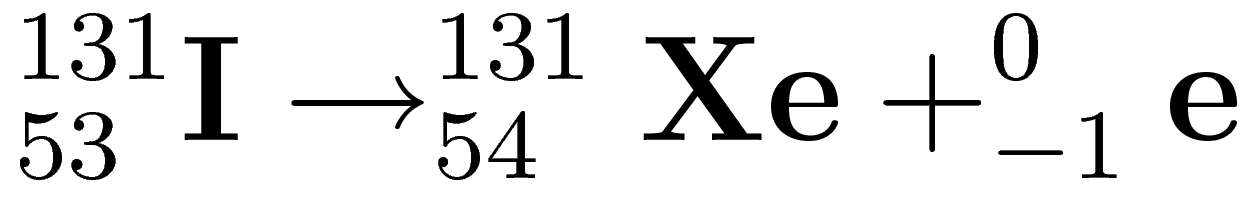
What is beta decay
300
How old is a sample that is 75% Nitrogen-14?
What is 11,460 years old.
300
Write an equation for the nuclear fission of Uranium-235 when bombarded with a neutron to produce xenon-144 and strontium-90
What is


300
What is the half-life of thorium-235?
What is 14 billion years (from your data package)
400
Isotopes have very similar ___________________ properties because the number of electrons is consistent between them.
What is chemical
400
Explain the changes that occur in the parent nucleus during alpha decay
What is a loss of 2 protons and 4 neutrons
400
What is the half-life of the isotope in the graph below?


What is 6 days
400
Why do nuclear fission & fusion reactions release so much energy?
What is a small amount of mass is lost from the nucleus and converted into a large amount of energy. More in fusion than in fission.
400
How are an alpha particle and a helium atom different?
What is a helium atom has electrons, but an alpha particle does not (hence the +2 charge)
500
Identify the number of neutrons, protons, and name of this isotope.


What is 143, 92, Uranium-235
500
Rubidium-87 undergoes beta decay. Write an equation representing this process.
What is

500
How long will it take a 200g sample of carbon-14 to decay to 12.5g?
What is 22,920 years
500
When do chain reactions get out of control?
What is when there are no measures in effect to control the number of particles (e.x. neutrons) released in each fission reaction.
500
How many protons does Plutonium-260 have?
What is 94.