Which species could be reduced to form NO2? a) N2O b) NO3– c) HNO2 d) NO
b) NO3–
Which changes could take place at the positive electrode (cathode) in a voltaic cell?
I. Zn2+(aq) to Zn(s)
II. Cl2(g) to Cl–(aq)
III. Mg(s) to Mg2+(aq)
I and II only
Consider the following reactions of three unknown metals X, Y and Z.
2XNO3(aq) + Y(s) → 2X(s) + Y(NO3)2(aq)
Y(NO3)2(aq) + Z(s) → No reaction
2XNO3(aq) + Z(s) → 2X(s) + Z(NO3)2(aq)
What is the order of increasing reactivity of the metals (least reactive first)?
X < Z < Y
What happens to the manganese in the following reaction? 2MnO4–(aq) + 5H2O2(aq) + 6H+(aq) → 2Mn2+(aq) + 8H2O(l) + 5O2(g)
It is reduced and its oxidation number decreases.
The standard electrode potentials for two metals are given below.
Al3+(aq) + 3e– Al(s) EO = –1.66 V
Ni2+(aq) + 2e– Ni(s)EO = –0.23 V
What is the equation and cell potential for the spontaneous reaction that occurs?
2Al(s) + 3Ni2+(aq) → 2Al3+(aq) + 3Ni(s) EO = 1.43 V
State which of the species is the strongest oxidizing agent
Fe2+(aq) + 2e– Fe(s) –0.45 V
H+(aq) + e– H2(g) 0.00 V
Br2(l) + e– Br–(aq) +1.07 V
Bromine/Br2
Which is the strongest reducing agent according to the spontaneous reactions below?
2Cr(s) + 3Fe2+(aq) -> 2Cr3+(aq) + 3Fe(s)
Fe(s) + Pb2+(aq) -> Fe2+(aq) + Pb(s)
Cr(s)
Deduce the order of reactivity of the four metals, cadmium, nickel, silver and zinc in the below equations and list in order of decreasing reactivity
Cd(s) + Ni2+(aq) → Cd2+(aq) + Ni(s)
Ni(s) + 2Ag+(aq) → Ni2+(aq) + 2Ag(s)
Zn(s) + Cd2+(aq) → Zn2+(aq) + Cd(s)
Zn > Cd > Ni > Ag
Nitrogen monoxide may be removed from industrial emissions via a reaction with ammonia as shown by the equation below.
4NH3(g) + 6NO(g) → 5N2(g) + 6H2O(l)
Deduce the oxidation and reduction half-equations and identify the oxidizing agent for the reaction.
Oxidation: 2NH3 → N2 + 6H+ + 6e–
Reduction: 2NO + 4H+ + 4e– → N2 + 2H2O oxidizing agent: NO
The amount of ethanol, C2H5OH(aq), in a person’s breath can be measured in an electrochemical cell. The relevant half-reaction equations are given below.
I CH3COOH(aq) + 4 H+(aq) + 4 e– → C2H5OH(aq) + H2O(l) E° = +0.58 V
II O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l) E° = +1.23 V
O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l)
The following equations indicate reactions that occur spontaneously.
Fe(s) + NiCl2(aq) → FeCl2(aq) + Ni(s)
Zn(s) + FeCl2(aq) → ZnCl2(aq) + Fe(s)
Ni(s) + PbCl2(aq) → NiCl2(aq) + Pb(s)
Which is the increasing order of the reactivity of the metals?
Pb < Ni < Fe < Zn
Which of the following metals will act spontaneously as reducing agents with silver iodide but not with silver sulfide?
A. Hg(l) and Cu(s)
B. Cr(s) and Al(s)
C. Ni(s) and Co(s)
D. Au(s) and Mg(s)
C. Ni(s) and Co(s)
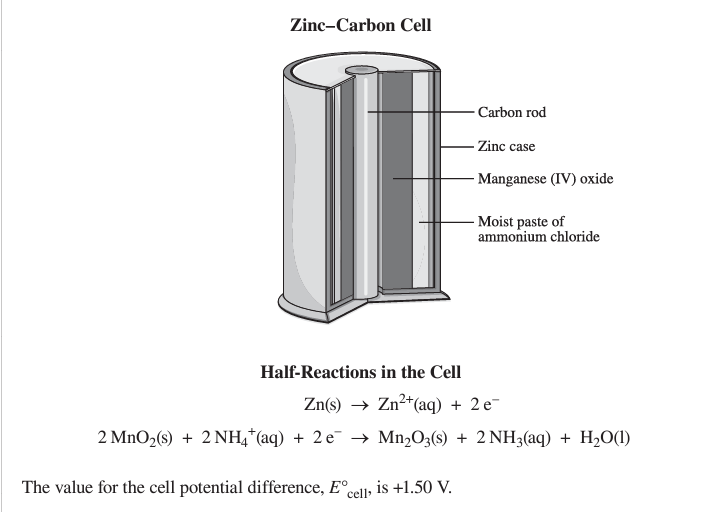
Complete the sentence:
A zinc–carbon cell is an example of i cell. The moist paste of ammonium chloride allows for the flow of ii .
a voltaic and ions
If the reference half-cell were changed from the standard hydrogen half-cell to the standard cadmium half-cell, then the reduction potential for the following equation would become
CH3COOH(aq) + 4 H+(aq) + 4 e– → C2H5OH(aq) + H2O(l) E° = +0.58 V
+0.98 V
A 150 mL sample of SO2(aq) required 31.5 mL of 0.0100 mol/L KMnO4(aq) to completely react. The concentration of SO2(aq) in the sample was
5.25 mmol/L