How many electrons are in an atom of krypton?
36
Which subatomic particles are located in orbitals?
electrons
Group 18 on the periodic table is classified as this two-word name.
noble gases
What type of bonding is present in the compound magnesium chloride?
ionic
Calculate the GFM of CO2.
44 g/mol
Endothermic reactions have a ___________
DeltaH
positive
To classify as hydrogen bonding, H must be bonded to ___, ___, or ___
F, O, N
As pressure increases, volume ___________
decreases
Solution is another word for a _____________ mixture
homogeneous
At equilibrium: rates are _______ and concentrations are _______
equal, constant
What does a substance with a pH of 3 classify as?
acid
oxidation refers to the _______ of electrons, and reduction refers to the _______
loss, gain
Name C5H12.
pentane
0.00340 has how many significant figures?
3
Which experiment led to the discovery that the atom consists of a positively charged nucleus surrounded by mostly empty space?
The Gold Foil Experiment
Name the two nucleons.
protons and neutrons
What are the only two liquids at STP?
bromine and mercury
In the following reaction 2Fe + O2 → 2FeO energy is ___________(released/absorbed) because a bond is __________(broken/formed)
released/formed Remember: BARF
What type of reaction does this classify as:
Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
single replacement
What does arrow B represent?
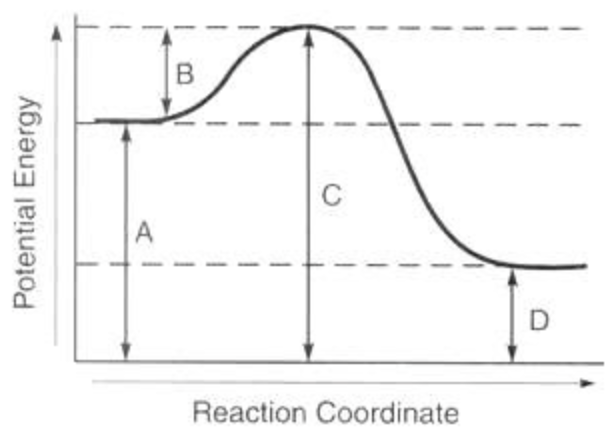
activation energy of the forward reaction
What is happening to kinetic and potential energy during this segment?
KE increases, PE stays the same
Real gases behave most like ideal gases under ________ (high/low) temp and ________ (high/low) pressure
high, low
filtration is used to separate ______________ from _____________
solids, liquids
If pressure is increased, the reaction shifts to the side with ______ moles of gas
less
give an example of an arrhenius base
starts with metal, ends with OH (ex. Ca(OH)2)
the oxidizing agent is the one that is __________, the reducing agent is the one that is ___________
reduced, oxidized
Name the following (be specific with numbers).
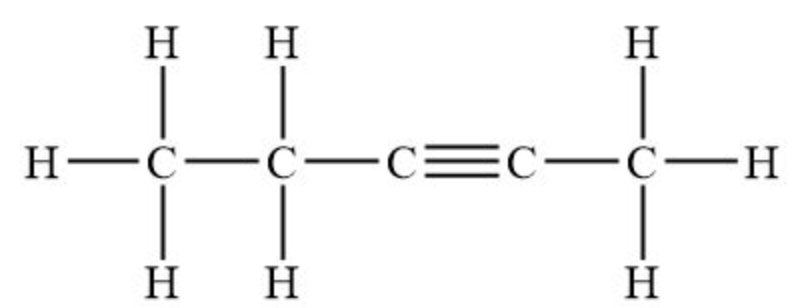
2-pentyne
Express 0.00000405 in scientific notation.
4.05 x 10-6
How many neutrons are in an atom of Rn-222?
136
Which two elements make up the bright line spectra below?
helium and hydrogen
Name all 7 diatomic elements.
Br2 I2 N2 Cl2 H2 O2 F2
Name the compound:
K2SO4
potassium sulfate
A 4.86-gram sample of calcium reacted completely with oxygen to form 6.80 grams of calcium oxide. This reaction is represented by the balanced equation below.
2Ca(s) + O2(g) → 2CaO(s)
Determine the total mass of oxygen that reacted.
1.94 g
What two things need to be true about colliding particles in order for an effective collision to occur?
sufficient activation energy and proper orientation
When pressure on the surface of ethanoic acid is 40 kPa, what is the boiling point?
90oC
The partial pressures of the three gases are 2.00 atm, 3.00 atm, and 4.00 atm, respectively. What is the total pressure inside the container?
9.00 atm
Explain, in terms of molecular polarity, why HCl is more soluble than H2 in H2O under the same conditions of temperature and pressure.
HCl and H2O are both polar so they are miscible
Given the equation representing a reaction at equilibrium:
N2(g) + 3 H2(g) ↔ 2 NH3(g) + energy
State a change that would cause the equilibrium to shift to the right.
dec. conc. of prods, inc. conc. of reacs, dec. T, inc. P
These three general categories for compounds can be electrolytes
acids, bases, and salts
What is the oxidation number of S in BaSO4
+6
This type of reaction involves the creation of carbon dioxide and water from reacting a hydrocarbon and oxygen together.
combustion
perform the operation with proper sig figs:
0.1 + 0.11 + 0.111
0.3
Which type of particle would be emitted to replace X in the following natural transmutation example?
13N --> 13C + X
a positron
How does the energy of an electron in the fourth shell compare to that of an electron in the second shell?
energy of the electron in the fourth shell is greater
Place the following in order from smallest to largest:
O−2, F−1, Mg+2, Na+1
Mg+2, Na+1, F-1, O-2
Write the formula for the following:
Lead (IV) oxide
PbO2
If a compound is 43.2% oxygen, what mass of oxygen is in 157 g of the compound?
67.8 g
In kJ, what is the amount of energy associated with the heat of reaction? INCLUDE A SIGN
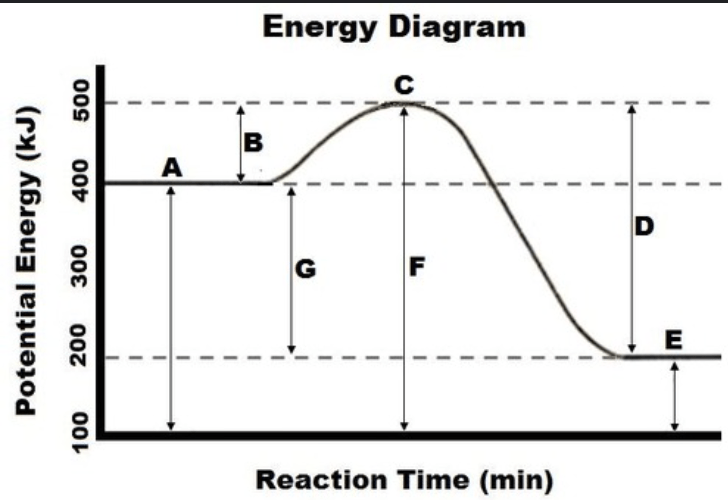
-200 kJ
Rank the IMFs from weakest to strongest
london dispersion, dipole-dipole, hydrogen bonding
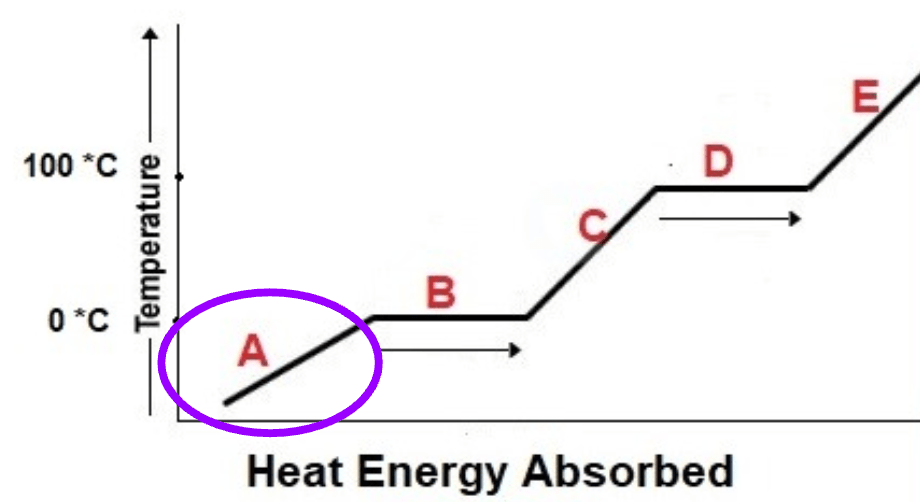
Which law is represented by the diagram below?
Charles' Law
Name the three ways to speed up dissolving solids in liquids.
increase temp, increase surface area, increase agitation
Given the following reaction at equilibrium:
N2(g) + O2(g) + 182.6 kJ ↔ 2NO(g)
When concentration of N2(g) decreases, equilibrium shifts _______, and concentration of O2 ________.
left, increases
What color will methyl orange be in a solution of KOH?
yellow
Which energy conversion occurs during the operation of a voltaic cell?
chemical to electrical
Write the general formula for saponification.
fat + base --> glycerol + soap
Read this measurement with proper sig figs
22.20 mL
How long does it take for 100g of potassium-42 to decay to 6.25g?
49.44 hours
State the electron configuration (Regents style) of the selenide ion, Se-2.
2-8-18-8
A compound has a molar mass of 90. grams per mole and the empirical formula CH2O. What is the molecular formula of this compound?
C3H6O3
Name the following:
Co(PO4)2
cobalt (VI) phosphate
Write and balance the following reaction using chemical symbols:
aluminum metal reacts with aqueous zinc chloride to produce zinc metal and aqueous aluminum chloride.
2Al(s) + 3ZnCl2(aq) --> 3Zn(s) + 2AlCl3(aq)
Name the three phase changes that lead to a decrease in entropy.
condensation (g-->l), deposition(g-->s), freezing (l-->s)
At standard pressure, the total amount of heat required to completely vaporize a 100.-gram sample of water at its boiling point is
226000 J
A 3.50 L sample of gas originally at 103 K shrinks to a size of 2.25 L at constant pressure. What is the new temperature (in Kelvin)?
66.2 K
Name the two ways to increase the amount of gas dissolved in a liquid.
● decrease temperature
● increase pressure
__________ equilibrium describes the phenomena that the forward and reverse processes always continue happening.
dynamic
The pH of a solution is 4. If the hydronium ion concentration is decreased by a factor of 10,000, what is the new pH?
8
Given the balanced equation representing the reaction occurring in a voltaic cell:
Zn(s) + Pb2+(aq) → Zn2+(aq) + Pb(s)
In the completed external circuit, the electrons flow from ______ to ______ (Name specifics, not general)
Zn(s) to Pb(s)
What is the name of the product of:
H – C ≡ C – H + H2 →
ethene
Convert 0.000426 kg to dg.
4.26 dg