Changes
In what units do you measure a liquid's volume?
In what units do you measure a solid's volume?
Liquids = liters
Solids = cm3
What is the formula for calculating density?
density = mass / volume
Which of the following represents a chemical change?
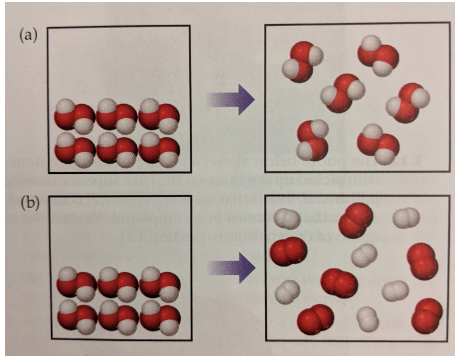
B
Is H2 an element or a compound?
Element
Every element has a unique number of protons, called the ______ number.
Atomic
Without using any numbers, demonstrate your understanding of mass and volume by providing an easy to visualize example of two objects of the same mass but different volumes.
5 grams of pennies vs 5 grams of feathers
What is the density of an object with a volume of 0.5 cm3 and a mass of 1.35 g?
2.7 g/cm3
Density, melting point, and boiling point are all examples of which property?
Physical properties
Most of the elements in our world are....?
metals / solid
How is the periodic table organized?
Without using any numbers, demonstrate your understanding of mass and volume by providing an easy to visualize example of an object with a large mass but a small volume.
A pool ball. A bowling ball. A gold nugget

6.3 g/cm3
Which of the following is NOT evidence of a chemical change?
A. Light given off
B. Makes sound
C. Changes in color
D. Change in size
D - change in size
The substance NaHCO3 (aq) has how many of each element?
1 Na
1 H
1 C
3 O
Rows = periods.
Columns = groups.
Why do some things float in water and some sink?
Float - density is less than 1 (aka density of water)
Sink - density greater than water
A 27.0 gram sample of an unknown metal is placed into a graduated cylinder and the water level rises from 11.5 mL to 14.5 mL. What is the identity of this metal?
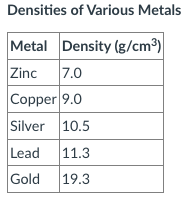
Copper
Reactivity is an example of which property?
Chemical
If there was a substance that had 1 sulfur, 3 oxygen, 2 hydrogen, and was a solid dissolved in water, what would its formula be?
SO3H2 (aq)
Technically, Hydrogens are written first, so:
H2SO3 (aq)
Which group is the most reactive metals?
Provide the order you would see the substances going from the bottom to the top.
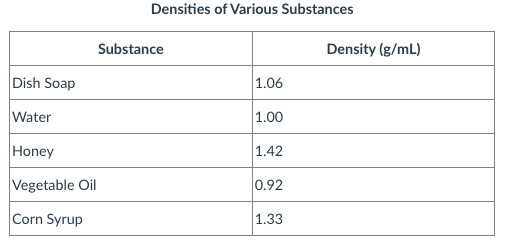
Bottom: Honey
4th: Corn Syrup
3rd: Dish Soap
2nd: water
top: veggie oil
Say that two graduated cylinders are both filled with 20.0 mL of water.
If 7.0 g zinc is placed in cylinder A and 9.0 g copper is placed in cylinder B, how does the amount the water level rises in each cylinder compare? What is the final volume reading in each cylinder?
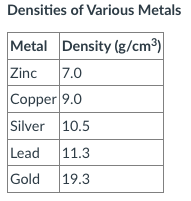
The final volume reading for zinc will be 21.0 mL.
The final volume reading for copper will be 21.0 mL.
Why? You added the same amount of metal as the density, so the water will increase by 1 cm3, or 1 mL.
Is dissolving a physical or chemical change?
Physical
If there was a substance that was a liquid and consisted of 4 oxygen, 1 manganese, and 1 potassium, what would its formula be?
O4MnK (l).
Technically, potassium is written first, so
KMnO4 (l)
Which group is chemically non-reactive?
Nobel Gases