If the atomic number is 19 what element is it?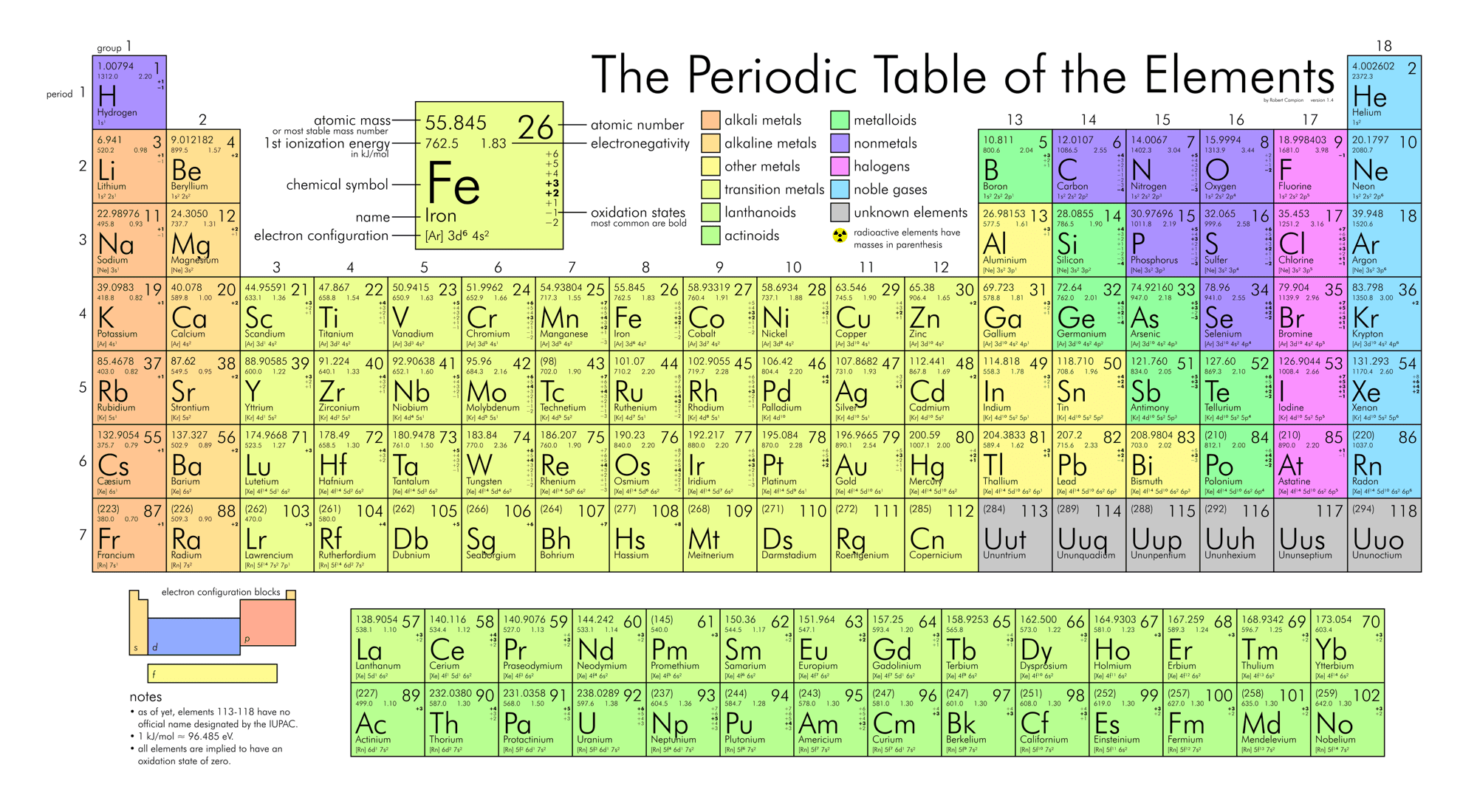
Potassium
What are the 3 main particles of an atom?
Protons, Neutrons, and Electrons.
How many atoms make up a molecule?
Two or more.
Which is bigger? Nucleus or atoms?
Atoms!
How are Protons charged?
Positively
How do the number of protons relate to the atomic number? 
The amount of protons is what the atomic number will be.
Where are the protons located in an atom?
They are located in the Nucleus.
NO!
What one is bigger? Neutrons or electrons?
Neutrons!
How are electrons charged?
Negatively
What is the atomic mass of silver/Ag? 
The atomic mass is 107.87.
Where are the electrons located?
Outside of the Nucleus in a cloud.
What atoms is H2O made of?
One Oxygen and two Hydrogen's.
Which is bigger? Neutrons or Protons?
Neutrons!
Are Neutrons charged?
No they are neutral
What is the atomic symbol of this element?
Mg
Where are the Neutrons located in an atom?
They are located in the nucleus.
Which is bigger? Molecules or atoms?
Molecules
Does the nucleus make up the whole atom?
No
What one moves? Electrons or Protons?
Electrons!
How are elements organized in the periodic table? 
What is the heaviest out of these three: protons, neutrons, or electrons?
Protons!!!!
Are molecules made of elements?
Yes!
Do electrons orbit the nucleus?
Yes
If you have 20 protons and the element is Calcium how many Neutrons do you have ?
?
20