Which has more particles?
1 mol of Fe or 1 mole of Ag
Neither
Isotopes of an element have different numbers of
Neutrons
What four letters represent the orbitals?
s, p, d, f
This formula shows the lowest whole number ratio of elements in a compound.
The number of protons in an element is known as its
atomic number
How many neutrons are in Carbon-14?
8 neutrons
the electrons in the valence shell of an element are ______________ away from the nucleus.
farthest.
What is the empirical formula of C6H9?
C2H3
A gold coin contains 3.47 x 1023 atoms of gold. What is the mass of the coin? Give your answer using 4 significant figures and the proper units!
113.5 grams/mol
What is the identity of the element in the spectra shown below?
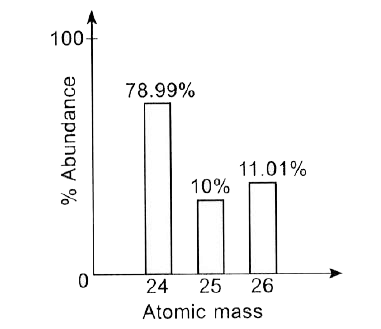
Magnesium
How many electrons can the d sublevel hold?
10
What is the empirical formula from the molecular formula C10H22?
C5H11
The % composition of Na in NaBr
What is 22.3%
How many neutrons are there in the most abundant isotope of Boron? 
5
What is the complete electron configuration of Ti?
1s2 2s2 2p6 3s2 3p6 4s2 3d2
Find the molecular formula of a compound with a molecular mass of 70g/mol and an empirical formula of CH2.
C5H10
The Principle that states that the lowest energy are filled first
Aufbau Principle
Isotope X-63 has a relative abundance of 69.2 %.
Isotope X-65 has a relative abundance of 30.8 %.
What is the average atomic mass of the element in amus?
63.616 amus
What is the shorthand configurations for Vanadium?
[Ar] 3d34s2
A compound composed of: 9.93% carbon, 58.6% chlorine, and 31.4% fluorine.
CCl2F2