How do you calculate formula mass?
1. Find molar mass of all elements
2. Multiply by how many there are
3. Add up
What are intermolecular forces?
Forces of attraction or repulsion between atoms
What are the physical properties?
Boiling, melting, and vapor pressure
What is melting point?
When a substance goes from solid to liquid
What is sublimation?
transition of a substance directly from the solid to the gas state, without passing through the liquid state
How do we calculate molar mass?
Use the atomic mass on the periodic table
List the forces in order of weakest to strongest
London dispersion
Dipole-dipole
Hydrogen bond
What is the trend with vapor pressure and forces
Stronger the intermolecular forces the lower the vapor pressure
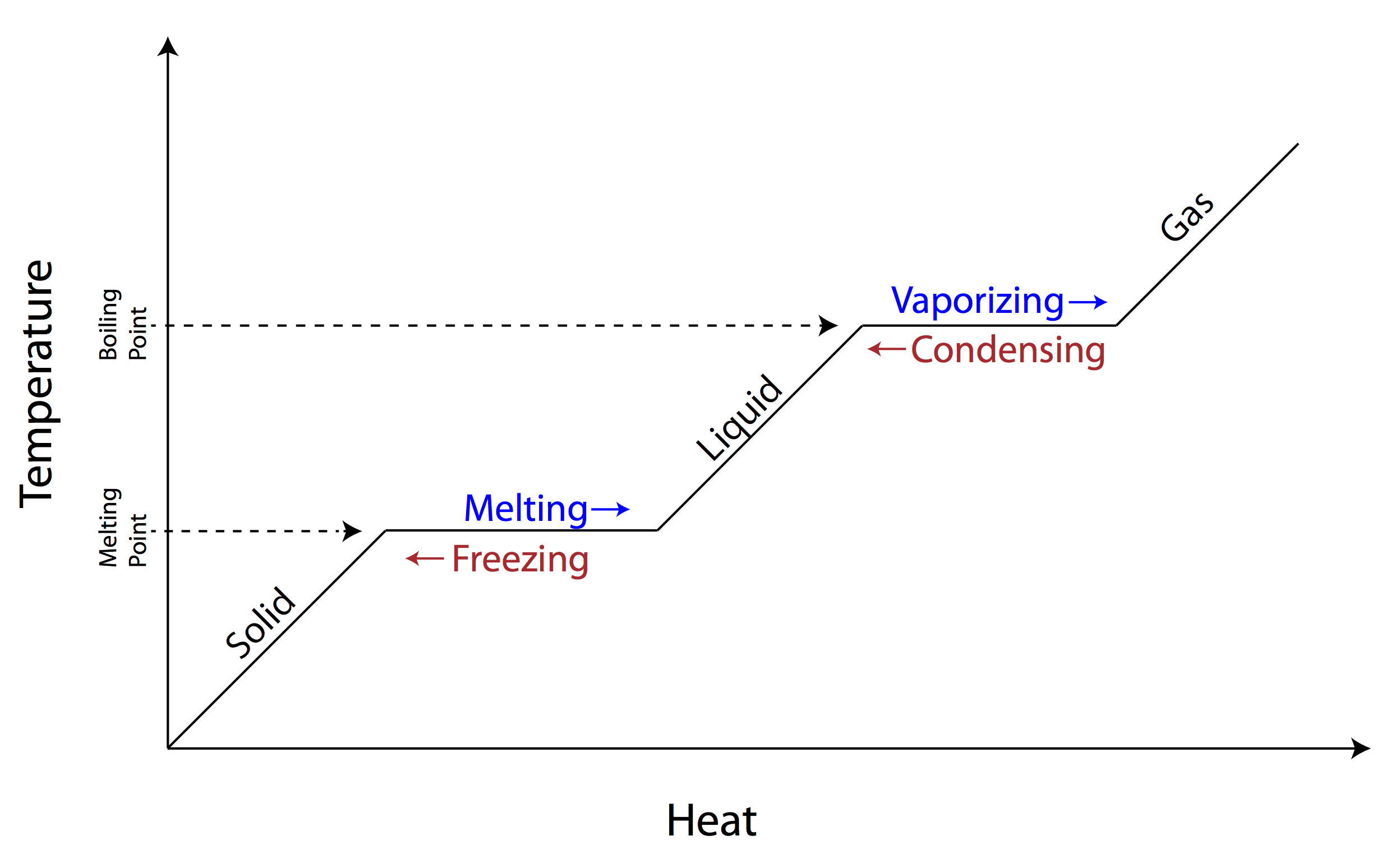
Explain in your own words what is occurring from liquid to gas change
Make sure to review what actually happens in phase changes!
Do like solutions dissolve yes or no?
YES
LIKE DISSOLVES LIKE
What is the units?
AMU (Atomic Mass Units)
What elements can create an hydrogen bond?
Oxygen, Nitrogen, Flourine
What is the trend with boiling point and forces
the stronger the forces the higher the boiling point

Rank melting point from highest to lowest
CH4 C2H5 C4H14 C5H8 C3H15
C4H14, C3H15, C5H8, C2H5, CH4
Find the formula mass for Co(ClO3)2 and identify the polyatomic ion
225.8 AMU
Identify the forces within CH4 (draw the structure)
TRUE or FALSE
Boiling point is liquid to vapor when heat is added
When the vapor pressure equals atmospheric pressure evaporation will begin
Vaporization is liquid to gas
True
False
True
Which type of crystal has the highest melting point?
ionic crystals
How do secondary forces/non polar or polar play into creating solutions?
Non polar and non polar will make a solution
polar and polar will make a solution
Find the formula mass for Ca3(PO4)2 and also identify the polyatomic ion
310.2 AMUFF
Identify the forces in HCl (draw the structure)
Bromine, Flourine, Carbon, Boron
Bromine
Molecular crystal
Covalent crystal
Ionic crystal
How do we know if a compound is polar or non polar?
such with the Lewis Structures
Symmetry!
Non polar=symmetrical around central atom
Polar=NON symmetrical (think of water and its lewis!)