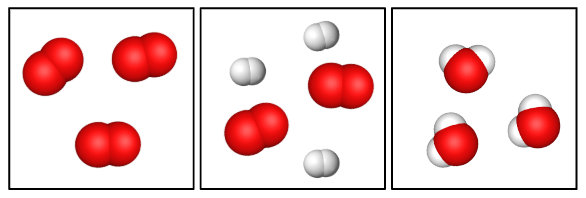
Which diagram represents a compound?
The filtration process is shown below. What particles will be collected in the beaker?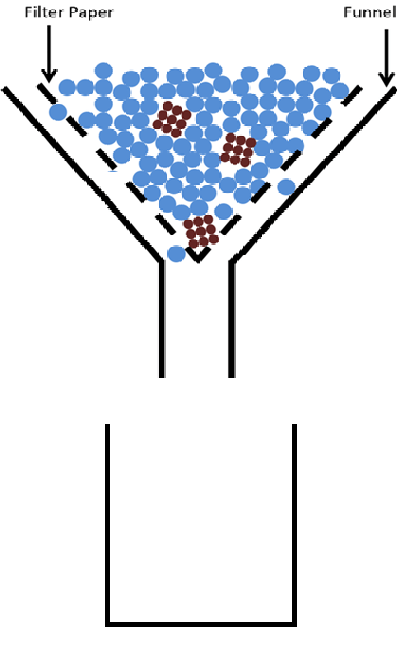
The blue particles will be collected in the beaker. The brown particles are too big to pass through the filter paper.
Intermolecular or Intramolecular?
The forces that hold atoms together within a molecule
Intramolecular
100 degrees Celsius to Kelvin?
373.15 K
A container holds two gases: Gas A (2.0 atm) and Gas B (1.5 atm). What is the total pressure?
3.5 atm
Three atoms of three different elements, He, Ne, and Ar, are shown below. What element do you expect Element #2 to be?
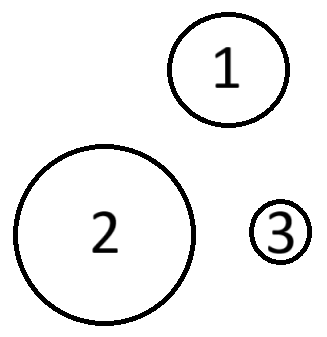
Argon
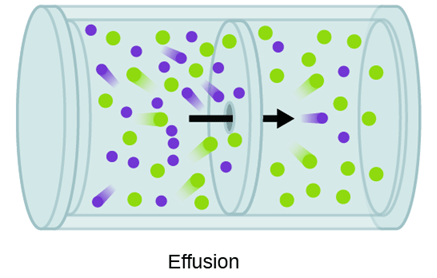
Based on the effusion separation process below, which particle (green or purple) would have a greater mass?
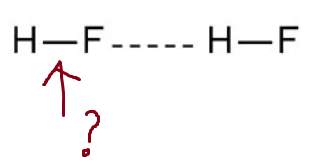
Intermolecular or Intramolecular?
The stronger force
Intramolecular
A gas has a pressure of 2.0 atm in a 3.0 L container. If the pressure increases to 6.0 atm, what is the new volume?
1.0 L
A mixture contains hydrogen, nitrogen, and oxygen. Their partial pressures are 0.4 atm, 0.6 atm, and 0.5 atm, respectively. What fraction of the total pressure is due to oxygen?
Xoxygen = 0.33
Is a glass of milk considered a pure substance?
Based on the chromatogram, which substances make up the Unknown sample?
Unknown sample is a mixture of A, B, and C.
Intermolecular or Intramolecular?
Intramolecular
A 3.0 L sample of gas is cooled from 350 K to 175 K. What is the new volume?
1.5 L
A container holds a gas mixture with a total pressure of 3.0 atm. The partial pressure of nitrogen is 1.2 atm, and oxygen is 0.8 atm. What is the partial pressure of argon?
PAr = 1.0 atm
An element made of two identical atoms has a molecular mass of 32amu. Draw the element. What is the element?
Oxygen
A very nonpolar solvent is used as the mobile phase for the chromatography experiment below. What color substance is the most attracted to the solvent? Is this color polar or nonpolar?

Purple, nonpolar
Which substance would has the strongest intermolecular forces?
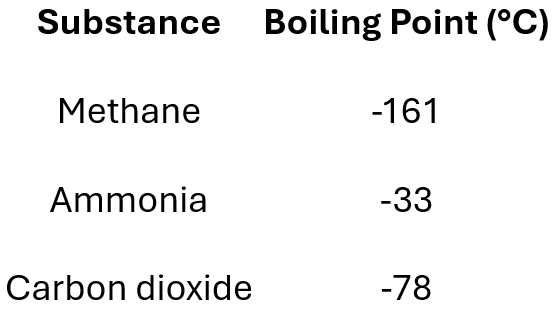
Ammonia
The pressure in an aerosol can is 2.0 atm at 25°C. If it is left in the sun and reaches 75°C, what will the new pressure be?
2.33 atm
A container has nitrogen at 300 K with a partial pressure of 0.9 atm. Oxygen in the same container has a partial pressure of 0.7 atm. If the temperature rises to 600 K, what is the total pressure assuming volume is constant?
Total pressure is 3.2 atm
Is water an element? Explain your answer.
Water is NOT an element, it is a compound. An element is a pure substance made of only one type of atom.
Which substance would have stronger intermolecular forces?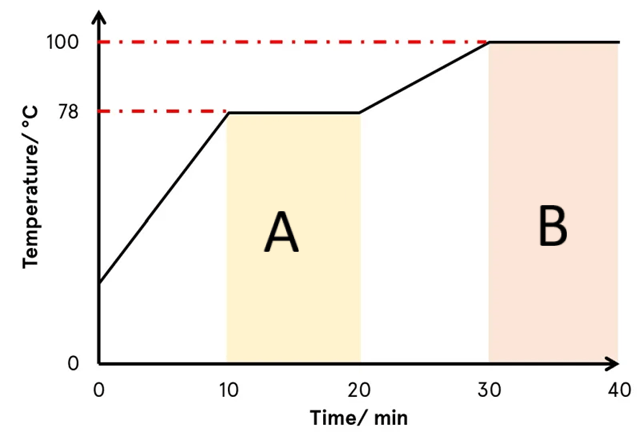
Substance B
Why do nonpolar substances generally have lower boiling points than polar substances?
Nonpolar substances have weaker intermolecular forces. Polar substances have uneven distributions of charge within their molecules, so one end is slightly positive and the other end is slightly negative. The uneven distribution of charge can attract oppositely charged molecule ends to create greater forces of attraction.
Why must temperature be measured in Kelvin for gas law calculations?
Kelvin is an absolute temperature scale where 0 K represents absolute zero, the point at which all molecular motion stops. Temperature must be measured in Kelvin for gas law calculations because gas laws (Boyle’s, Charles’s, Gay-Lussac’s, and the Combined Gas Law) rely on a proportional relationship between temperature and volume or pressure.
A container contains 57 particles of a gas mixture. What is the total pressure of the container if 12% of the gas mixture has a partial pressure of 1.2 atm?
Total pressure is 10 atm