Which block is the element Germanium (Ge) located in?
p-block
Which of the following functional groups is characteristic of ketones?
-COOH
-COOR
-C=O
-CHO
-C=O
Which of these atoms is most electronegative?
Cl
Mg
Br
Na
Cl
What's the complete electronic configuration for Arsenic?
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p³
How would you name this structure? 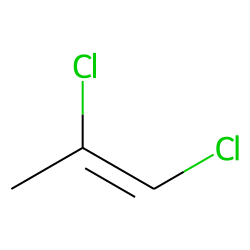
1,2-dichloropropene
Arrange the following in order of increasing atomic radius (smallest first): Na, Si, Cl. Explain your reasoning.
Cl < Si <Na
Na, Si and Cl are all period 3 elements and atomic radius decreases from left
to right across a period because of increasing nuclear charge and the electron
shielding remains constant.
What would you see when sodium is added to water?
A gas is given off
The temperature of the water increases
A clear, colourless solution is formed
I and II only
I and III only
II and III only
I, II and III
I, II and III
How would you name this structure?
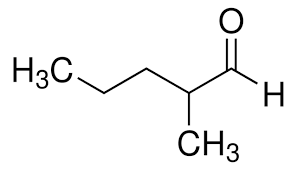
2-methyl pentanal
What would this structure be called?

Diethyl ether
What is the oxidation number of oxygen in H2O2?
Write a balanced chemical equation, complete with state symbols for the reaction of potassium and water
2K(s) + 2H2O(l) → 2KOH(aq) + H2(g)