What is the difference between qualitative and quantitative observations?
qualitative = based on qualities
quantitative = based on quantities
When are zeroes significant?
1. Sandwich zeroes
2. After decimal AND after non-zero digits
What is the purpose of dimensional analysis?
Converting numbers into different units, without changing their value
The exponent will be:
+ if you started with a _____ number (> 1) or
- if you started with a _____ number (< 1)
+ = big
- = small
Part 3: Classify the following mixtures as homogeneous or heterogeneous:
1. Sand
2. Pizza
3. Paint
4. Saltwater
1. HE
2. HE
3. HE
4. HO
A property of matter that depends on the amount of matter present would be considered a(n) _________property
extensive
True or false:
8.4 - 8.5 - 8.3 - 8.2 are precise values
TRUE
How many sig figs are in 0.400?
3
912 cups to liters
215 L
Write in scientific notation:
78, 700,000,000
7.87 x 1010
True or false:
Saltwater is an heterogenous mixture
FALSE
Classify the following properties as extensive or intensive:
1. Mass
2. Density
3. Viscosity
1. Extensive
2. Intensive
3. Intensive
Kim tells his mom he can't go to school today to take his chemistry test because he is running a temperature of 208.89 K. What is his temperature in degrees Fahrenheit?
-83.67°F.
How many significant figures are in 1234567890?
9
0.06 tons to grams
60 000g
Write in standard notation:
5.01 x 10-2
0.0501
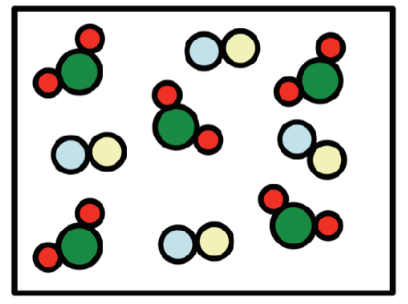
This is...
A = Element
B = Compound
C = Mixture of elements
D = Mixture of compounds
E = Mixture of elements and compounds
D = Mixture of compounds
True or False:
Odor is a physical property
True or False:
An odor change is a chemical property
TRUE & TRUE
Luis measured a substance and his result was 20g. If the known mass is 30g what is his percent error?
50%
What is the density of a ball if it's mass is 35g and it's volume is 23.6 cm3
1.5 g/cm3
0.315 gal to tablespoons
79.5 tbsp
Write in scientific notation:
0.008800000
8.800000 x 10-3
11. You are given a container filled with clumps of iron and sand. You can separate the iron from the sand using a magnet. This makes this a:
A. homogeneous mixture
B. compound
C. heterogeneous mixture
D. solution
C. heterogeneous mixture
Classify the following as a chemical change (C) or a physical change (P).
a. Chopping wood
b. Burning wood
с. An apple oxidizing
d. Steam from a shower
P - C - C- P
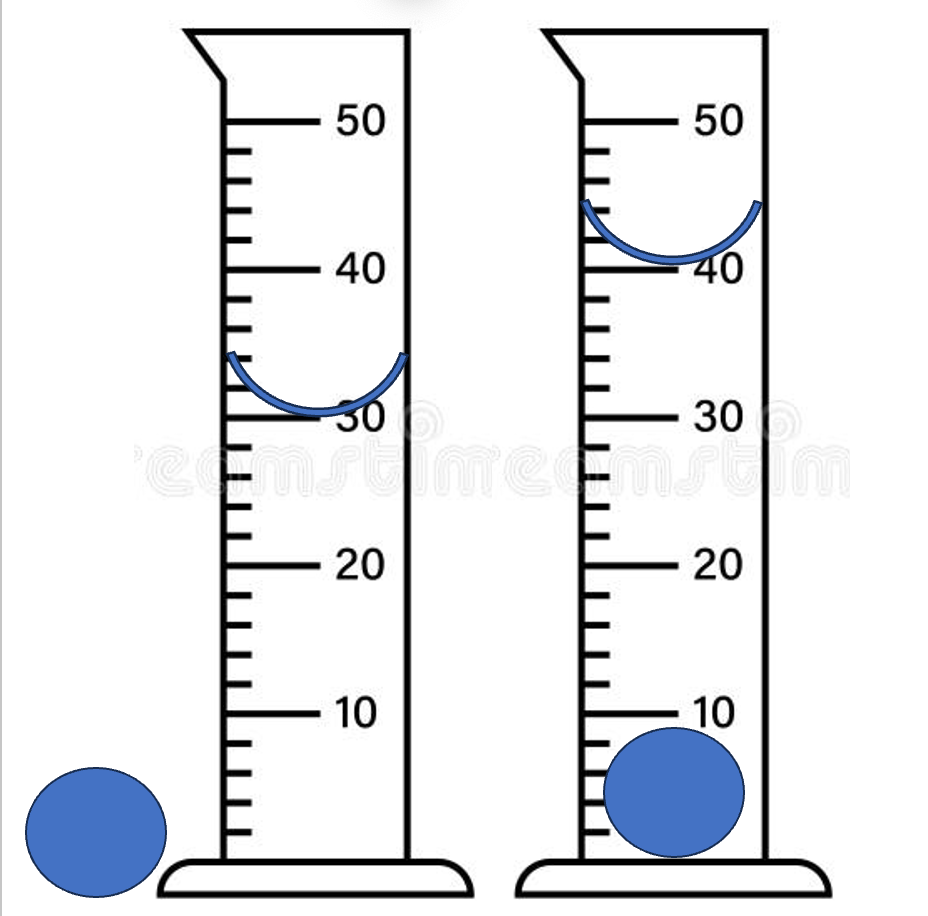
What is the density of the ball if it's mass is 45g
4.5 g/ml
 Mary measured the length of a rod four times. If that's her data, what is the average of her results?
Mary measured the length of a rod four times. If that's her data, what is the average of her results?
17.2
Which of the following measurements would be considered the smallest?
A. 10,000 ug
B. 1,000 hg
C. 100 cg
D. 10 g
10 000 ug
Write in scientific notation:
17,200 000 000 000 000 000 000 000
17.2 x 1025
Classify the following as an element, compound, homogeneous mixture or heterogeneous mixture:
Iron (Fe)
Carbon dioxide (CO₂)
Salad with dressing
Table sugar (C₁₂H₂₂O₁₁)
Nitrogen (N₂)
Saltwater
- Iron (Fe) – Element
- Carbon dioxide (CO₂) – Compound
- Salad with dressing – Heterogeneous mixture
- Table sugar (C₁₂H₂₂O₁₁) – Compound
- Nitrogen (N₂) – Element
- Saltwater – Homogeneous mixture
During a lab experiment, a student combines 50 g of sulfur with 100 g of iron to form iron sulfide. After the reaction is complete, the student notices that the total mass of the iron sulfide produced is 150 g.
Explain why the mass of the products equals the mass of the reactants in this reaction.
LAW OF CONSERVATION OF MASS