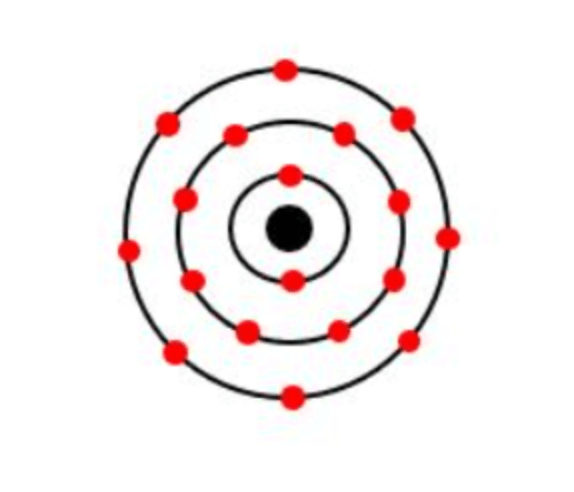
The number of electrons in the first energy level.
What is 2?

(Without looking at the periodic table)The element that looks like this on the periodic table.
What is Cobalt?

The name of this group of elements.
What are the Noble Gases?
This creates an ionic bond.
This is
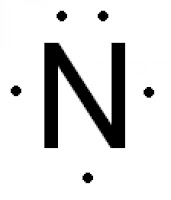
What is the Lewis Dot Diagram of Nitrogen?
When something goes straight from a solid to a gas.
What is sublimation?
The elements that bond with Noble Gases.
What are none?
The color of dye that Arsenic was used to make.
What is green?
The charges of protons, neutrons, and electrons.
What is positive, neutral, and negative?
The atomic mass of an element is equal to this.
What is the number of protons plus the number of neutrons?
Electronegativity moves in this direction on the periodic table.
What is from left to right?
A positive ion.
Hydrogen and helium need this in their valence shell to be happy.
What are two electrons?
The two parts of a solution.
What are a solute and solvent?
Two types of chemical reactions.
What are
synthesis, decomposition, single displacement, double displacement, or combustion?
The energy that is stored in an object.
What is potential energy?
The scientist who thought all matter was made up of fire, air, earth, and water.
Who is Aristotle?
Example of an element with a chemical symbol that does not "match" it's name.
What is Iron (Fe), Lead (Pb), Tin (Sn), Mercury (Hg), Tungsten (W), etc?
The group and period number that Hydrogen is in.
What is group 2, period 1?
A covalent bond occurs between these two types of elements.
What are a two nonmetals?
In a water molecule, this element is the central atom of the Lewis Dot Diagram.
What is Oxygen?

Silicon is this type of element, which acts as a semiconductor.
What is a metalloid?
True or False: New atoms are created and destroyed in a chemical reaction.
What is false?
How electrons emit light.
What is the electrons jump to a higher energy level then release energy as they return to their ground state?
This number of electrons orbiting Potassium.
What is 19?
Names of two radioactive elements.
(Without looking at the periodic table)
What are Uranium, Plutonium, Radium, Polonium, etc?
The periodic table is full of information on these.
What are atoms that make up all matter?
A compound with three or more elements.
What is a polyatomic?
Aside from Groups 1 and 2, elements and compounds want to have this to be complete.
What are 8 electrons in their valence shell?
The definition of polarity.
What is the unequal sharing of electrons in a compound?
A decomposition reaction.
What occurs when one reactant breaks down into two or more products?
Dry ice is this many degrees colder than regular ice.
What is 109 degrees?
An energy level represents this.
What is the area in an atom where an electron will be found?
Atoms of the same element with different numbers of neutrons.
What are isotopes?
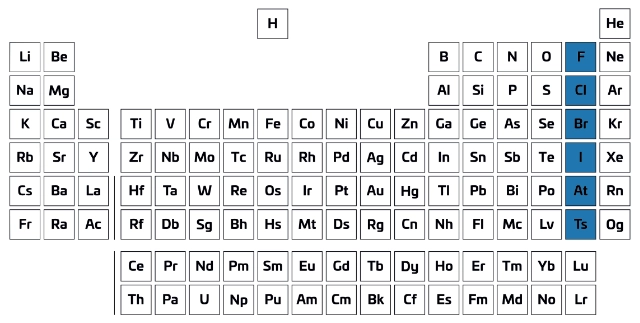
Group 17 on the periodic table (shaded area).
Hint: it means salt forming
What are Halogens?
The chemical name for PO43-
What is phosphate?
The chemical name for CCl4
What is Carbon tetrachloride?
The density of water.
What is 1g/ml?
The five main signs that a chemical reaction has taken place.
What is light, temperature change, gas production, color change, and formation of a precipitate.
The natural color of Neon.
What is reddish-orange.