Describe the term soluble.
(of a substance) able to be dissolved
Describe the term concentrated.
solution with high concentration (solute/ solution) compared to other substances
Describe the term melting.
change of a solid into a liquid when heat is applied
Describe the term opaque.
not transparent; impenetrable to light; not allowing light to pass through.
Describe the term insoluble.
something that will not dissolve in a solvent
Label the structures in the lungs.
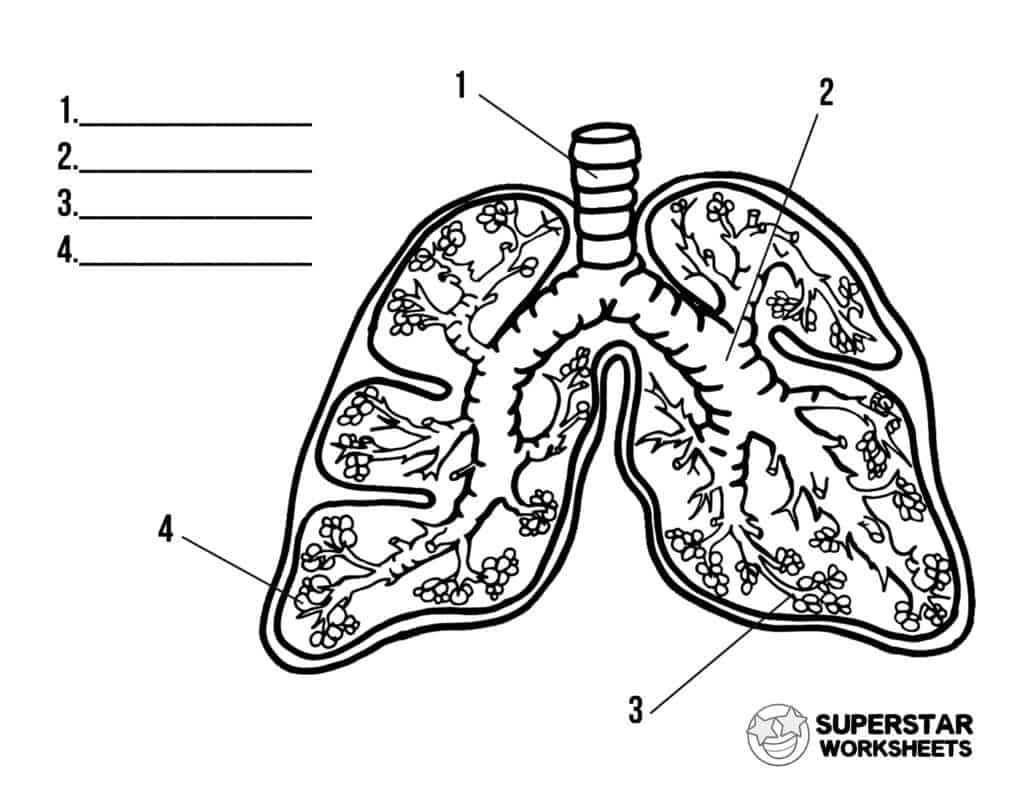
1. Trachea
2. Bronchus
3. Bronchiole
4. Alveoli
Why does carbon dioxide diffuses from the capillary to the alveoli?
air in the alveoli has lower concentration of carbon dioxide than the blood in the capillary
Describe the appearance and functions of red blood cells.
Disc-shaped (biconcave), red, no nucleus
Carry oxygen from the lungs to the body and carbon dioxide from the body to the lungs.
Describe the appearance and functions of white blood cells.
Various shapes, larger than red blood cells, have a nucleus
Help fight infections and provide immunity.
Describe the appearance and functions of plasma.
Clear, yellowish liquid
Transports nutrients, hormones, and waste products throughout the body.
What happens to diaphragm, intercostal muscles, and lungs when you breathe in?
intercostal muscles and diaphragm contracts and lungs expands
What happens to diaphragm, intercostal muscles, and lungs when you breathe out?
intercostal muscles and diaphragm relaxes and lungs contracts/compresses
How does oxygen gets absorbed into the blood stream?
it diffuses through the thin wall of the alveoli
What are released during respiration?
carbon dioxide, heat/energy, water
How does increasing the temperature affects the solubility of a substance?
solubility increases with temperature
Differentiate solute and solvent.
solute is the one being dissolved
solvent in the one that dissolves the solute
50 grams of sodium chloride solution
50 g of sugar dissolves in 100g of water at 15˚C.
How much sugar will dissolve in 200g of water at 15˚C?
100g of sugar
Which substance is the most and least soluble?
Blue and red
To which direction with the block move to?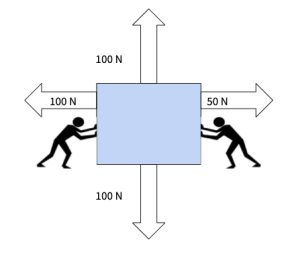
to the right
Moegi is in the car travelling at a speed of 20m/s. How long will the car take to travel 100m?
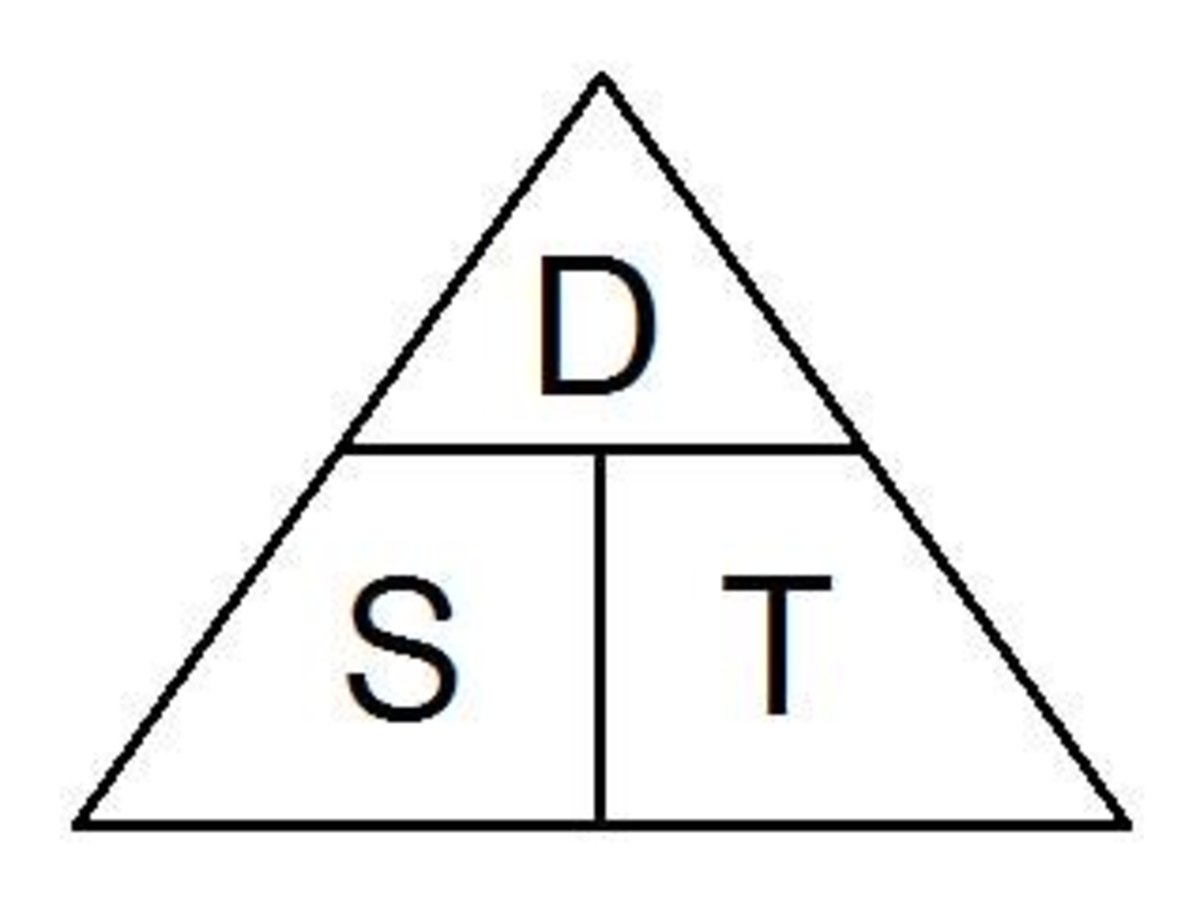
100/20 = 5s
At what speed does the car travel from point 1 to point 2?

60/1.5 = 40m/s
What is the speed of the train on the way back from point 3 to point 4?

60/2 = 30 m/s
A pulling force of 50N is needed to open a door. The distance from the door handle to the door hinges (the pivot) is 1m. What is the moment caused by the pull on the door?
50x1 = 50Nm
Your friend weighs 500N and sits at a distance of 2m from the pivot of a seesaw. You weighs 400N. Where should you sit to make the seesaw balanced?

(500 x 2)/ 400 = 2.5m away from the pivot