as atomic radius of a metal decreases, the reactivity of the metal ________
decreases
This is the term for the minimum energy required to remove the first electron from a neutral atom.
first ionization energy (IE1)
As atomic radius of a non-metal decreases, the reactivity of a non-metal ______
increases
Why is F the most electronegative element?
Because it has the smallest atomic radius for a non-metal and it only needs to gain 1 e- to fill its outer shell
Compared to lithium, sodium has a lower first ionization energy primarily because _______
it has a larger atomic radius and therefore a weaker nuclear pull on the valence e- (they both only need to lose 1 electron)
Elements with a low electronegativity also have a low __________
ionization energy
Draw the Lewis structure for PCl3
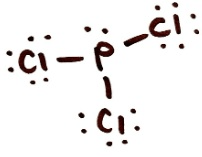
Draw the Lewis structure for CuS
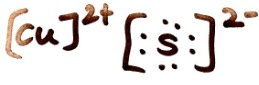
This type of element typically has low electronegativity because it tends to donate electrons rather than attract them.
metals
A chlorine atom will have a much higher second ionization energy than the first because _____
the 2nd e- would be removed from a full valence shell which means it will be very unstable
An element with a large atomic radius is likely to have a ____ electronegativity value
low
An element with 6 or 7 valence electrons would likely have _____ electronegativity and ____ ionization energy
high; high
_______ increases down a group due to the addition of electron shells, even though the number of protons also increases
atomic radius
Both O and F are in the same period so why does F have a higher electronegativity?
fluorine has more protons (and therefore a smaller atomic radius), increasing its nuclear pull on valence electrons
Despite gaining both protons and electrons across a period, atomic radius decreases because ___________
increasing + nuclear pull on the electron orbits
Rank the following in order of LEAST to MOST electronegative: F, Ca, Cl, K, Br,
K, Ca, Br, Cl, F
Which is the correct structure for SO4 2-? Why?
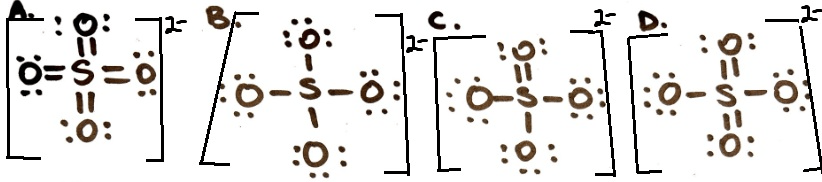
D because F.C on 2 of the oxygens is -1 and everything else is neutral
Rank the following in order of MOST to LEAST reactive: P, As, F, Cl,
F, Cl, P, As
Removing a third electron from magnesium requires significantly more energy than the first removal because ____
Mg will readily give up 2 electrons in order to have a full outer orbit. However, if a 3rd e- is removed, now it is removed from a full orbit and this makes Mg very unstable
Rank the following in order of MOST to LEAST first ionization energy: F, He, Cl, Fr, K,
He, F, Cl, K, Fr