How many protons, neutrons and electrons are there in an atom of 197Au ?
The superscript represents the mass number (protons + neutrons)
197Au has 79 protons, 79 electrons, and 197 -79 = 118 neutrons
Draw the Lewis structure for CO2

False
"Like dissolves like"
Which type of intermolecular force is the strongest?
Atomic size increases down a group and decreases across a period in the periodic table.
Therefore, the relative atomic size of Mg, Be and Ba is as follows:
Ba>Mg>Be
Draw the Lewis Structure for MgCl2
A Mg2+ ion will be isoelectric to which element?
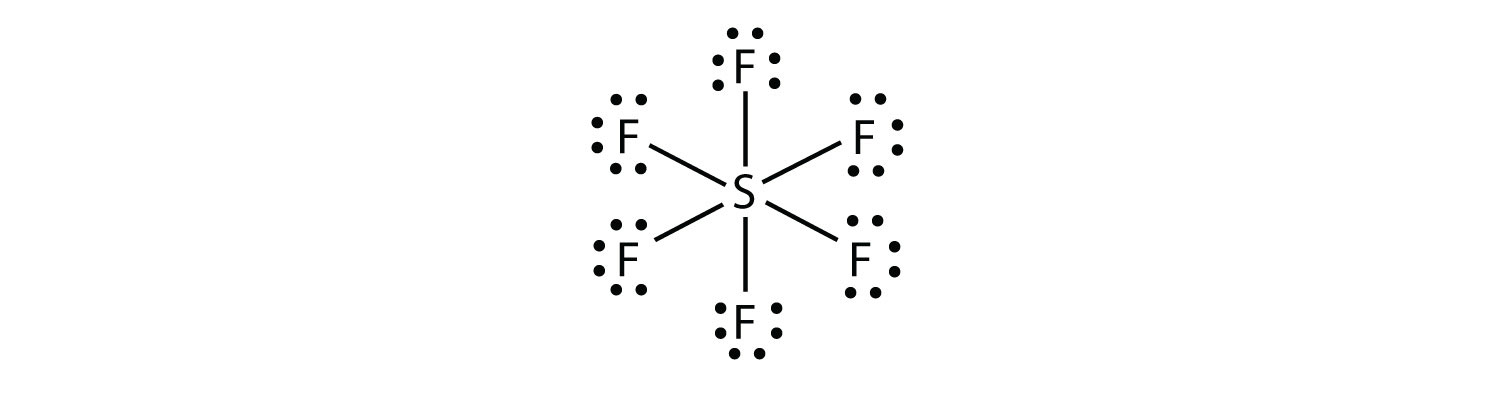
Draw the Lewis Structure for SF6

Calculate the △EN and determine the type of bond for C-O (ENc = 2.5, ENo = 3.5)
△EN = 1.0
1.0 > 0.4 ∴ this is a polar bond

The amount of energy required to remove the outermost electron from an atom or ion in the gaseous state is called the __________________________.
Ionization Energy
metal
nonmetal
Draw the Lewis structure for AlCl3
Is CH3Br polar or nonpolar?
Which molecule will have the highest melting point: NH3 or H2O?
H2O
Draw the Bohr-Rutherford Diagram for Fluorine

Which compound will have the higher boiling point, MgO or H2O?
Draw the Lewis structure for PCl3
Is BF3 polar or nonpolar?
What type(s) of intermolecular forces are present in CH2O?
Dipole-dipole and london dispersion