What is the definition of an Arrhenius Base?
Compound that reacts with water to form OH- ions
Is a compound with a pH of 11.5 a base or an acid?
Base
What are the products of the following reaction?
NaOH + HCl ->
NaCl + H2O
Name the piece of lab equipment that contains the titrant
Burette
Is normal precipitation neutral, slightly basic, or slightly acidic?
slightly acidic (pH = 5.9)
What is the definition of an Bronsted-Lowry acid?
Compound is a proton donor
Which pH value below represents the most acidic compound:
1, 5, 3
pH = 1
What are the products of the following reaction:
Mg(OH)2 + HCl ->
H2O + MgCl2
Phenoltphthalein is ______________ in basic solutions and ____________ in acidic solutions.
pink, colourless
What do we call precipitation that has a pH value below 5.9?
acid precipitation
Which beaker represents a strong dilute acid?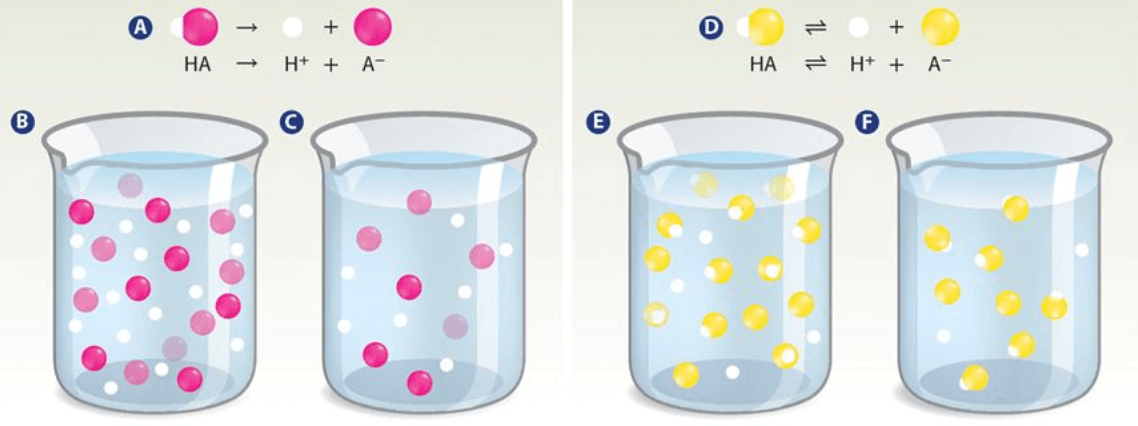
Beaker C
What is the pH of an acidic solution that has a hydronium (H3O+) concentration of 10-3 mol/L
pH = 3
Consider the balanced chemical equation below:
HCl + NaOH -> NaCl + H2O
What volume of 0.150 mol/L HCl is needed to neutralize 0.025 L of 0.140 mol/L NaOH?
VHCl = 0.023 mol/L
This is the point in a titration when the indicator changes colour.
What is the End Point?
Name a compound that contributes to acid precipitation.
nitrogen dioxide (NO2)
Sulfur dioxide (SO2)
What is the difference between a strong acid and a concentrated acid?
Strong acid completely ionizes in water
Concentrated acid there are a lot of solutes dissolved in solution
What is the hydronium concentration of a solution that has a pH of 10?
[H3O+] = 10-10 mol/L or 1 x 10-10 mol/L
Consider the following chemical equation:
HNO3 + NaOH -> NaNO3 + H2O
20.00 mL of HNO3 reacts with 15.00 mL of 0.150 mol/L NaOH. What is the concentration of HNO3?
[HNO3] = 4.5 x 10-5 mol/L
Why do we perform acid-base titrations?
Acid-base titrations are used to find the concentration of an acid or base
Show how NO2 contributes to acid precipitation using a chemical equation.
2NO2 + H2O -> HNO3 (strong acid) + HNO2 (weak acid)
List 2 properties of basic solutions and 2 properties of acidic solutions
Basic: Tastes bitter; Has a pH greater than 7; turns red litmus paper blue; pink when using phenolphthalein indicator; conducts electricity; corrodes tissues but not metals; does not react with metals; does not react with carbonates
Acidic: tastes sour; has a pH less than 7; turns blue litmus paper red; colourless when using phenolphthalein indicator; conducts electricity; corrodes tissues and metals; reacts with metals to produce hydrogen gas; reacts with carbonates to produce carbon dioxide gas
What is the pH of a solution that has a hydronium (H3O+) concentration of 3.5 x 10-3 mol/L ?
pH = 2.5
Consider the chemical reaction below:
2 NaOH + H2SO4 -> Na2SO4 + 2H2O
What concentration of 40.00 mL NaOH is needed to neutralize 20.00 mL of 0.4 mol/L H2SO4?
[NaOH] = 0.4 mol/L
An average volume of 60.00 mL of 0.10 mol/L LiOH solution is required to neutralize 25mL samples of HBr in a titration reaction. What is the concentration of HBr?
[HBr] = 0.24 mol/L
Name two ways humans can reduce the amount of acid precipitation
Plant more trees
Reduce vehicle use
Use more energy efficient vehicles
retrofit older homes to eliminate air leaks