Describe the mathematical relationship of wavelength, frequency, and energy.
As wavelength increases, frequency and energy decrease
(wavelength is inversely proportional to frequency and energy)
(frequency and energy are proportional)
Draw the orbital diagram for oxygen
___ ___ ___ ___ ___
1s 2s 2p
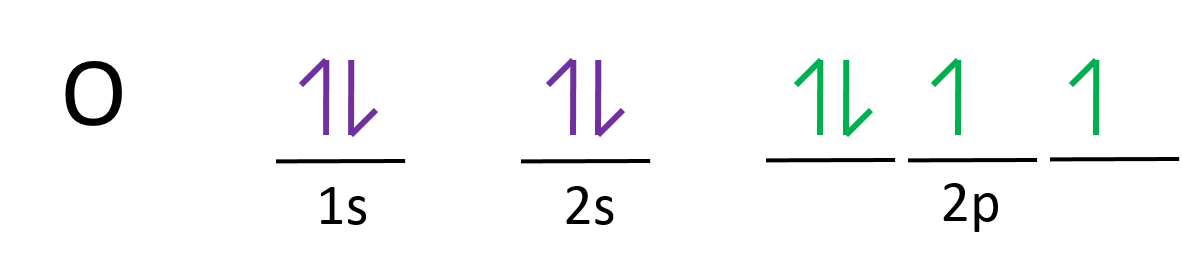
What element(s) do not have a short hand electron configuration?
H and He
A light wave has a frequency of 5.88 x 1014 Hz.
The wavelength of light is how many nanometers?
HINT: The speed of light is 3.00 x 108 m/s. Remember you will have to convert from meters to nanometers. 1 nanometer = 1 x 109 meters.

510 nanometers
How many electrons can fit in the n = 3 energy level?
18
When will an atom emit a photon?
When an electron moves from a higher energy level to a lower energy level (closer to the nucleus). The energy of the photon emitted will be equal to the difference in quantized energy levels the electron moved.
# of orbitals in the 3rd energy level (n=3)
9 orbitals (s contains 1, p contains 3, and d contains 5)
What goes into the square brackets when writing a short hand electron configuration?
The noble gas that precedes the element.
Calculate the energy, in joules, of a photon of red light that has a frequency of 3.73 x 1014 Hz.
The speed of light is 3.00 x108 m/s. 1 meter = 1 x 109 nanometers. Planck's constant is 6.626 x 10-34 Js.

2.47 x 10-19 J
What model of the atom do we use to describe the absorption and emission of light, even though it is not perfect and not the most recent one?
The Bohr model
What is the difference between a continuous, emission, and absorption spectra?
continuous: whole spectrum of color (ROYGBIV)
emission: shows only the wavelengths (lines) of light emitted by an element/compound
absorption: Inverse of emission spectrum shows all colors except those emitted by the
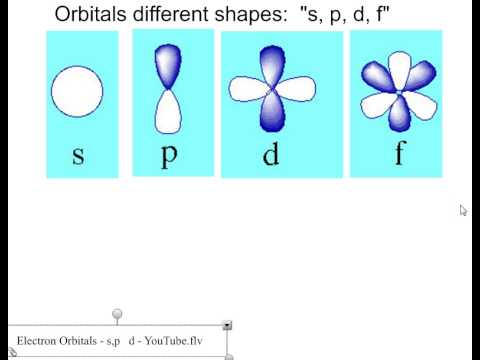
Draw the shapes of the s, p, d, and f
What is the long hand electron configuration for Ar?
1s22s22p63s23p6
Calculate the wavelength (in nanometers) of the hydrogen emission line that corresponds to the transition of the electron from the n = 3 to the n = 1 state.
Rydberg's constant = 2.18 x 10-18 J
Planck's constant = 6.626 x 10-34 JS
Calculate the frequency of the wave.

2.93 x 1015 Hz
What does it mean that light has a dual nature?
Light sometimes acts as a wave and sometimes acts as a particle.
What element(s) is the unknown sample made of?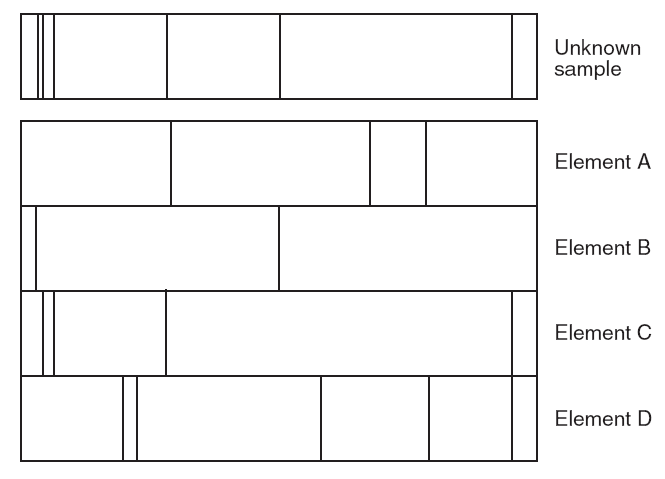
Element B and Element C
The number of electrons in n=4 energy level
32 e- (2 from s, 6 from p, 10 from d, 14 from f)
there's an equation for this
Write the electron configurations (noble gas notation) for sodium and carbon.
Na: [Ne] 3s2
C: [He] 2s22p2
Lithium shows the photoelectric effect at a wavelength of 432 nm. What is the mass of this photon?
5.11x10-36 kg
Which electron transition cannot happen?
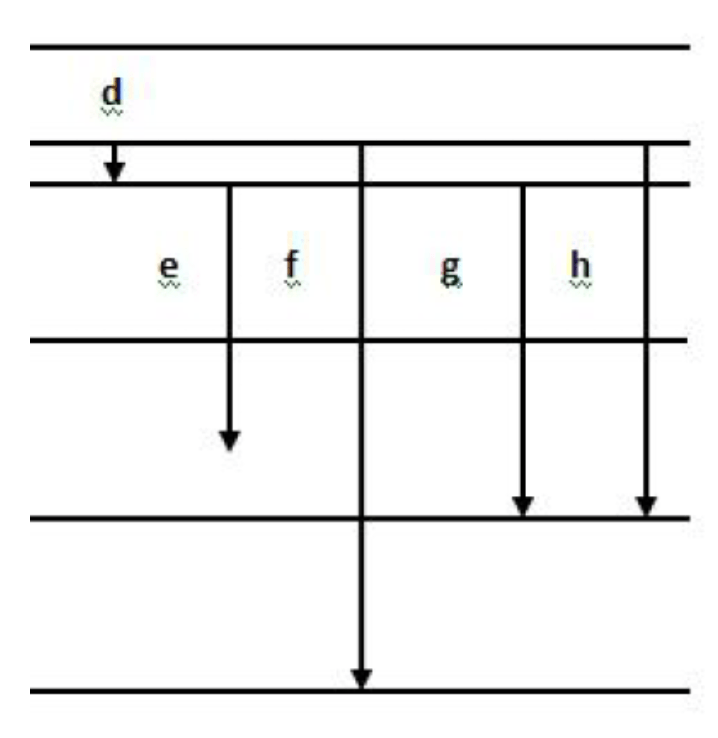
e
An electron has to "fall" or "jump" to a specific energy level because energy is quantized.
Which of these waves is traveling the fastest?
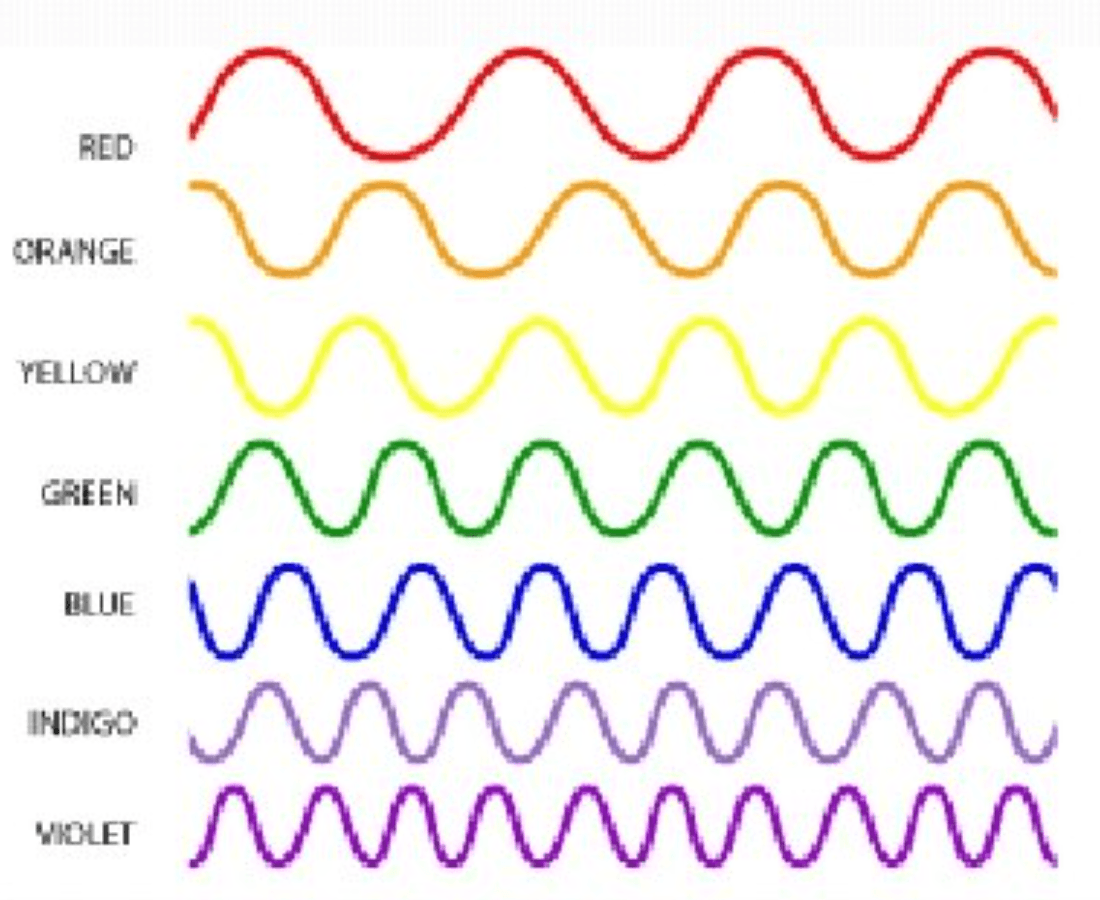
They are all traveling at the same speed
3.00 x 108 m/s
The number of valence electrons in an atom of As?
As has 5 valence electrons (in group 15 or 5A)
[Ar]4s²3d104p³
In what energy level are the valence electrons of zirconium and how many does it contain.
[Kr]5s24d2
2 valence electrons, 5th energy level
If Harry and his Nimbus 2000 (combined mass = 75.0 kg) are flying at maximum speed (44.7 m/s), what would be its wavelength?
1.98x10-37 m
How many orbitals exist in each of these sublevels?
s, p, d and f
s = 1 orbital
p = 3 orbitals
d = 5 orbitals
f = 7 orbitals
What kind of electron transitions absorbs light and which emit light?
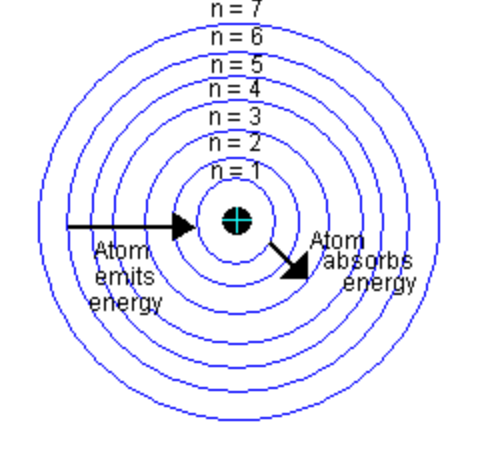
An electron moving to a higher energy level absorbs light.
An electron moving to a lower energy level emits light.
The number of valence electrons contained by the noble gases.
8, except He which has 2
Provide the element name, period, group, and block for the following electron configuration:
[Kr]5s24d8
Element is Pd
period: 5
group: 10
block: d
What is the wavelength (in nm) and type of radiation of the photon emitted from a hydrogen atom when its one electron drops from the 4th energy level to the 1st energy level?
97.3 nm, UV radiation
How many orbitals do each of these energy levels have?
n = 1
n = 2
n = 3
n = 4
n = 1 has one orbital
n = 2 has four orbitals
n = 3 has nine orbitals
n = 4 has sixteen orbitals