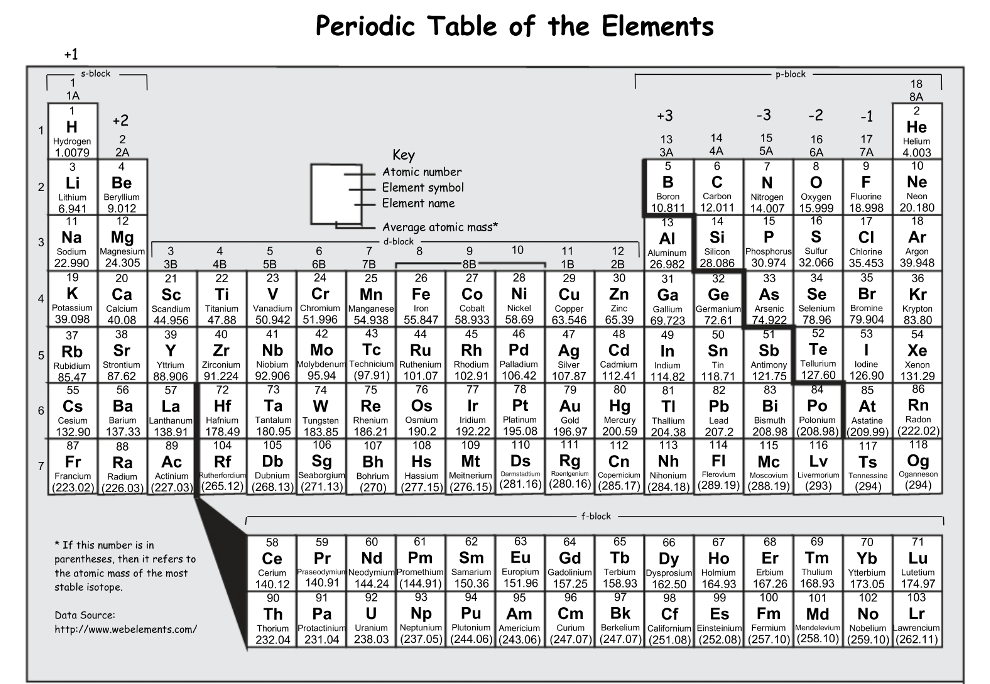
_______ are non reactive because they have a full octet/duet of valence electrons.
noble gases
Which of the following elements is the most reactive: Sr, Y, V
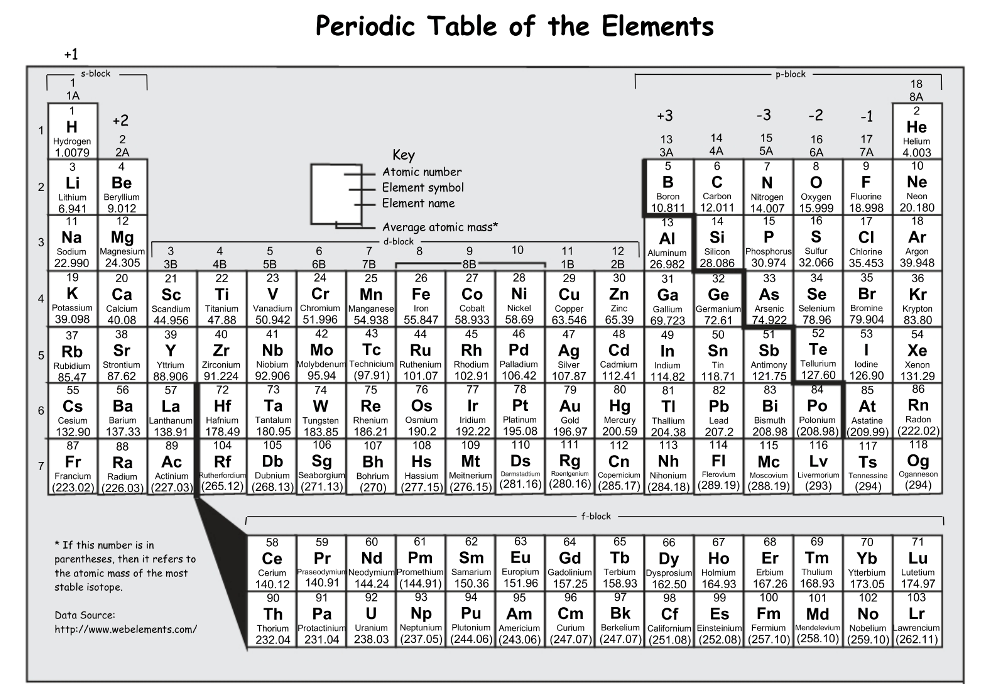
Sr, it would have the highest reactivity of the metals listed because it is the closest to Fr
Describe Alkali Metals
Found in Group , except Hydrogen
very reactive because they only have 1 valence electron.
•Properties:
•Soft (low density)
•Shiny
•Good conductor
•Reacts violently with water
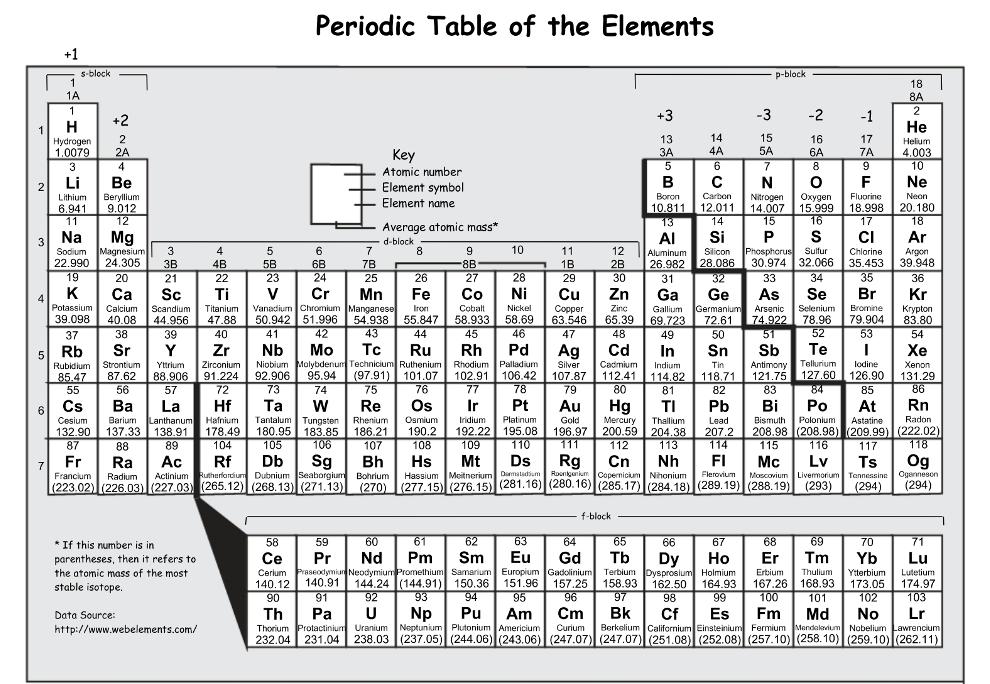
Sort the following into metals, nonmetals, and metalloids: Br, Na, B, Mn, Te, O
Metal: Na & Mn
Nonmetal: Br & O
Metalloid: B & Te
The number of electrons for sulfur is ________.
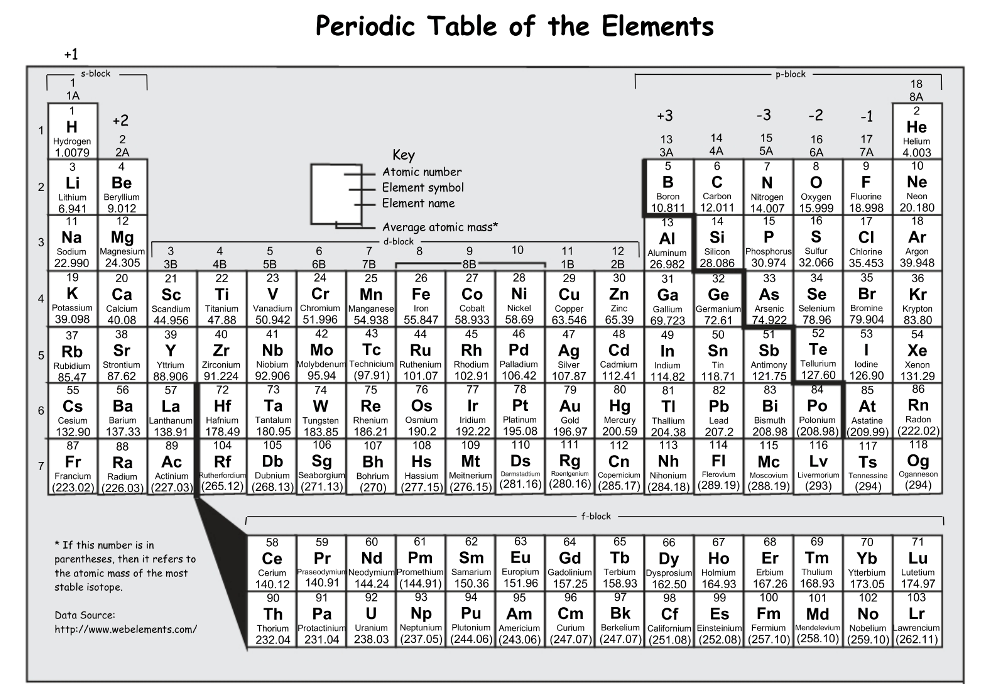
16
Which has a larger atomic radius: Ti or Y
Y: atomic radius increases down a group and decreases across a period, we also know that helium has the smallest radius so the further an element is to He the larger it will be
Describe Noble Gases
Make up group 18 of the periodic table
Properties:
•Inert
•Unreactive (stable)
•Colorless, odorless gases
•Full outer energy level
•8 VE (except for He)
Do the following elements have similar properties: P, Bi, Sb
Yes (all elements are in group 15, elements in the same group tend to have similar chemical and physical properties)
The number of valence electrons in an atom of As?
As has 5 valence electrons (in group 15 or 5A)
[Ar]4s²3d104p³
Which has a larger atomic radius: Ti or Y
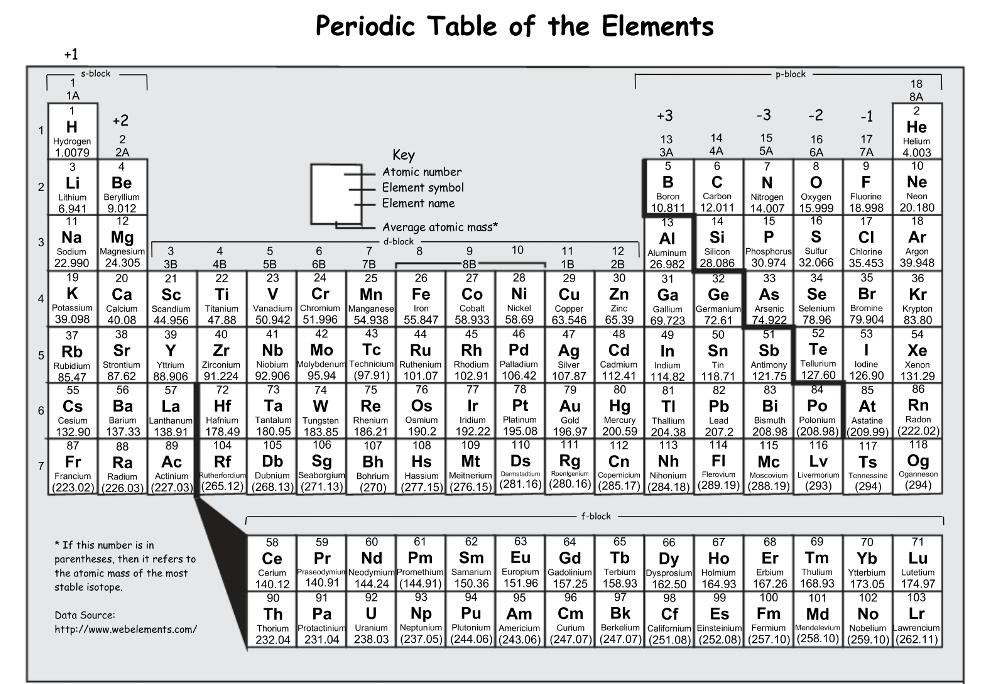
Y: atomic radius increases down a group and decreases across a period, we also know that helium has the smallest radius so the further an element is to He the larger it will be
Describe Metalloids
•neither metals or nonmetals.
•They have properties of both.
•They are known as semi-conductors.
Form a staircase on the periodic table
Where are the representative (main group) elements located?
s & p blocks
The number of electrons for Gallium is______.
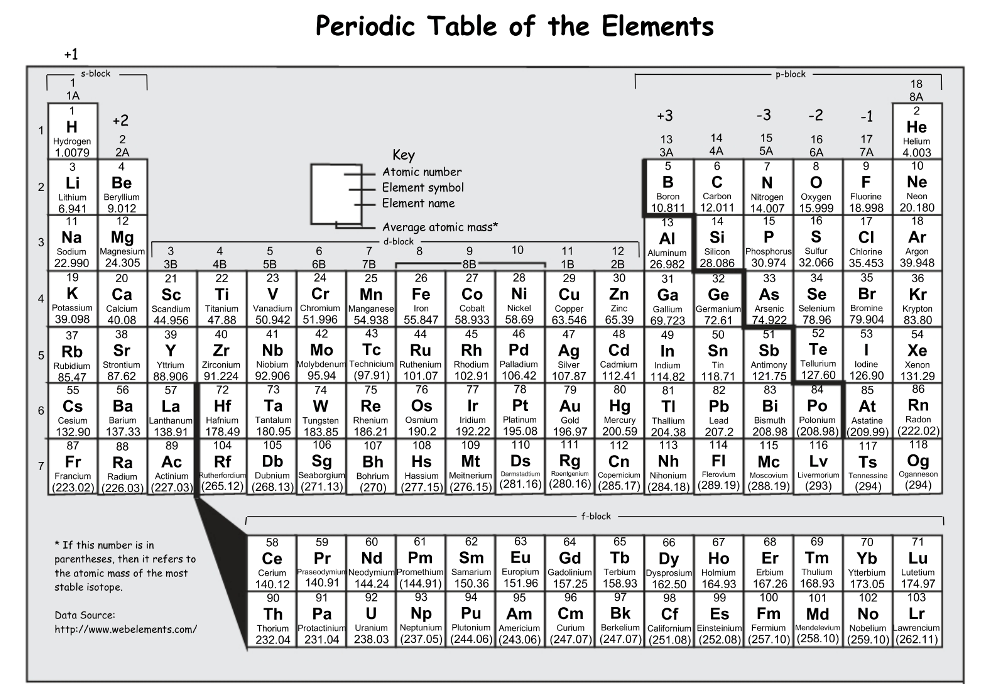
31
Which element has a lower ionization energy?
S or Si
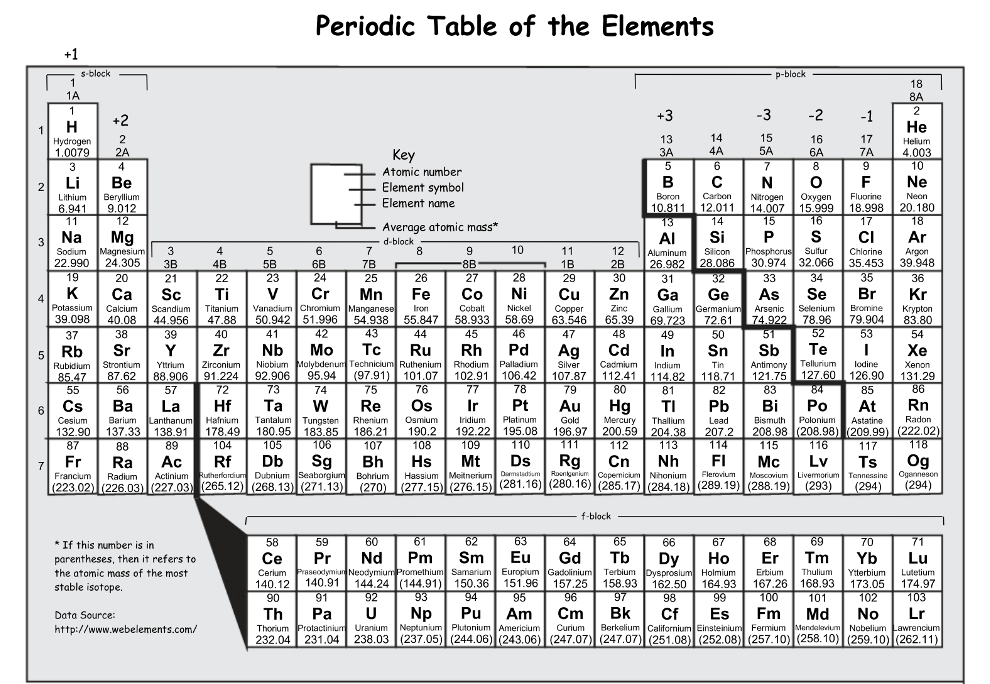
Si: ionization energy increases across a period, both elements are in period 3 so the element further to the left would have a lower ionization energy (also Si is further from He)
Describe Alkaline Earth metals
Found in Group 2
2 valence electrons
•Properties:
•Less reactive than alkali, but still react
•Combine to form compounds
•Good conductor
Do the following elements have similar properties: W, Pt, Au, Hg
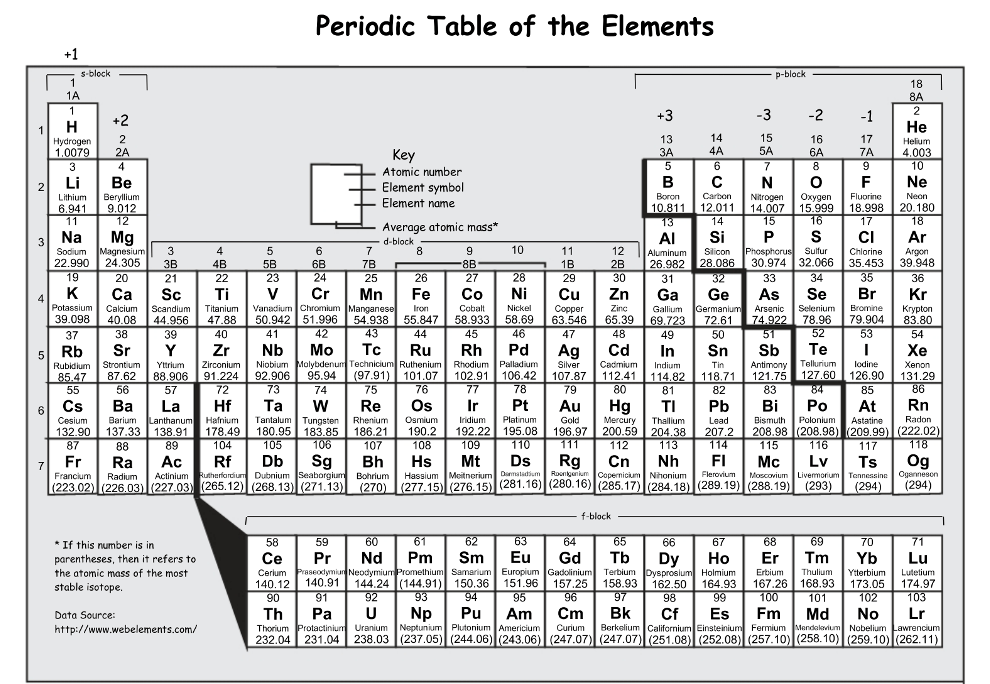
No (they are all in period 6, but not a part of the same group)
How many electrons does zirconium have? And how did you find this number?
40 electrons, look at the atomic number
What element has the higher electronegativity?
N or P
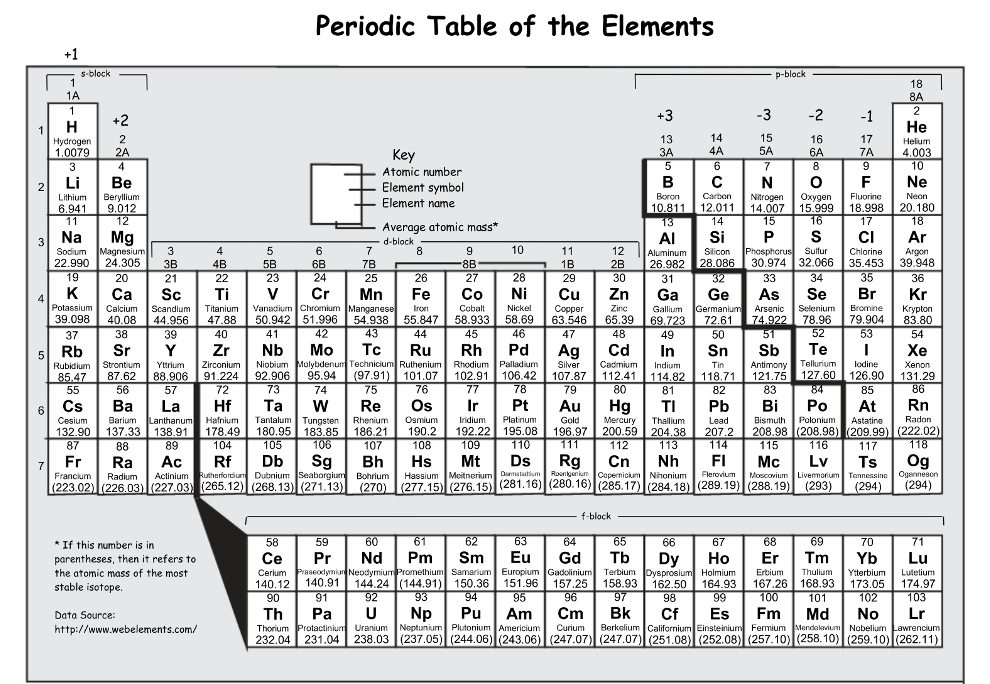
N (nitrogen), electronegativity tends to decrease down a group
(the closer an atom is to fluorine on the periodic table the more electronegative the atom is)
Describe Transition Metals
•Located in groups 3-12 on the periodic table
•Properties:
•Much less reactive
•Good conductor
Name 4 elements that would have 3 electron shells.
Anything that is located in Period 3
The number of valence electrons contained by the noble gases.
8, except He which has 2
Which of the following elements is the most reactive:
P, Ar, Br
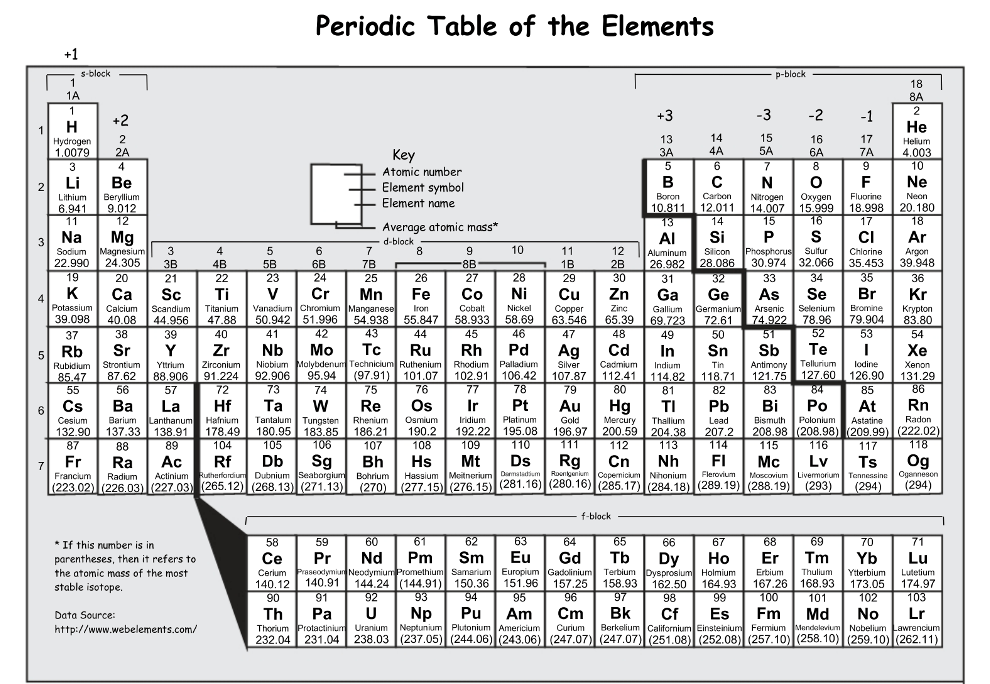
Br, it would have the highest reactivity of the nonmetals listed because it is the closest to F after recognizing the Ar is automatically the lease reactive since it is a noble gas
Describe Rare earth metals
•Located at the bottom of the periodic table
•Properties:
•silver, silvery-white, or gray metals.
•have a high luster, but tarnish readily in air.
•Good conductors of electricity
If I have a mass of 78.96 AMU and 6 valence electrons, what element am I?
Selenium