The number of neutrons in an iron atom
What is 30
(55.8 round to 56 for mass number. 56-26 = 30)
The number of bonding electrons in this diagram:
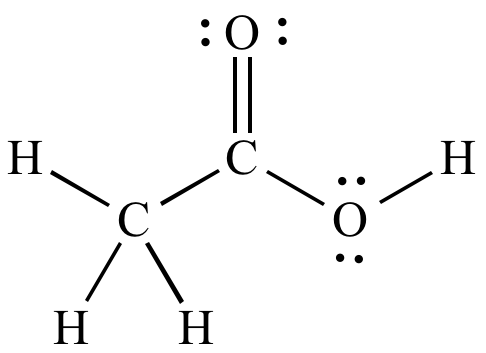
What is:
16 electrons
(each bond = 2 electrons)
The name of Mn2S3
What is:
Manganese (III) sulfide
The number placed in front of a chemical formula in a balanced chemical equation
Energy and matter are allowed to enter/exit a system
What is:
Open system
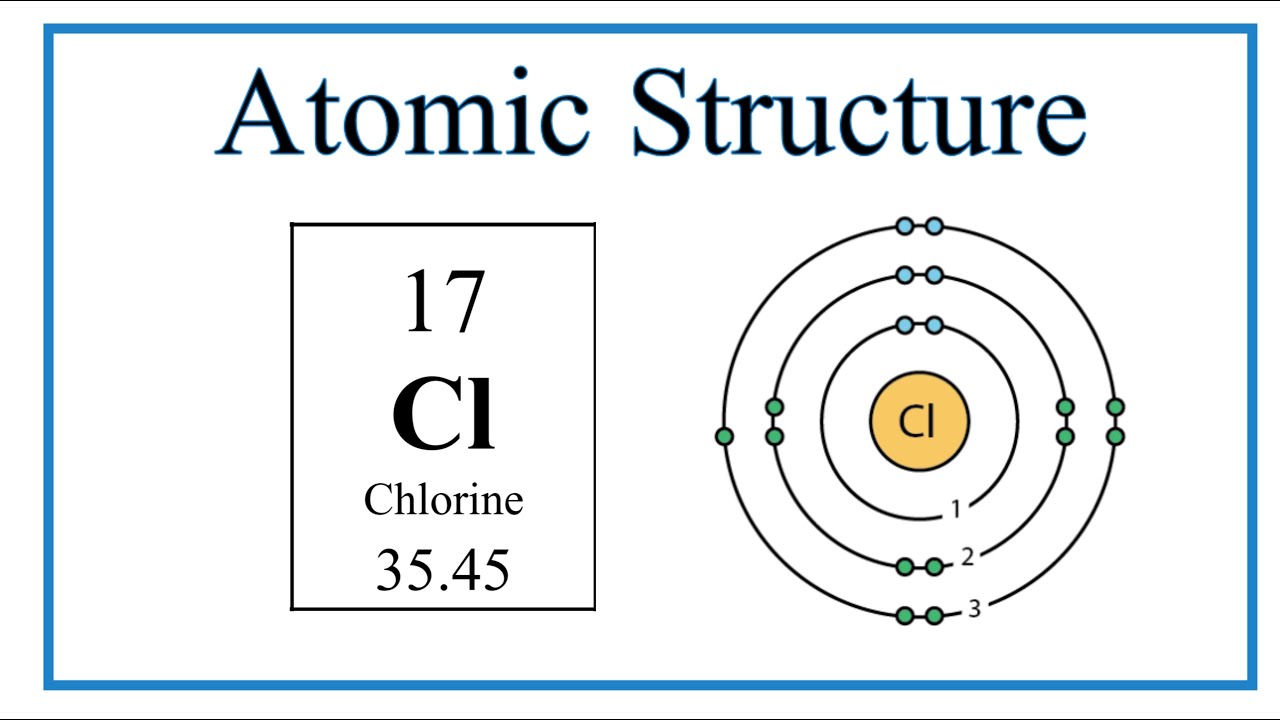
The number of energy shells in Chlorine
3 energy shells
(count each period as 1 energy shell)
A property of covalent compounds
- involves a non-metal and a non-metal
- sharing of electrons
- gases and liquids at room temperature
- lower melting points than ionic bonds
- most are insoluble and stay as molecules
- don't conduct electricity
The name for N4O9
What is:
tetranitrogen nonoxide
Write and balance the equation:
potassium chlorate --> potassium chloride + oxygen
2KClO3 --> 2KCl + 3O2
Re-write the equation with the heat term included as part of the equation:
2Na(s)+Cl2(g)→2NaCl(s) , change in enthalpy = -411.2kJ
2Na(s)+Cl2(g)→2NaCl(s) + 411.2kJ
Only one chance!
Which of the following have the same number of total electrons:
1) Na, Mg, Al
2) Li+, Na+, K+
3) K+, Ca2+, Ar
4) N3-, O2-, F
3) 18 electrons total
The driving force behind the formation of chemical bonds
What is:
Stability
The name of HF
What is:
hydrofluoric acid
(Recall: elemental acids - hydrogen __ide --> hydro__ic acid)Curious George finds a bottled drink in the hat of The Man with the Yellow Hat. He wanted to find out if the drink is water (pH =7). Using some methyl red to one sample, the solution turned red. To another sample, he used methyl orange and the solution turned red. What is the pH of the solution (give a range)? And can it be determined as water?
Methyl red: pH range for colour change is 4.8-6.0, as pH increases, the colour goes from red to yellow
Methyl orange: pH range for colour change is 3.2-4.4, as pH increases, the colour goes from red to yellow
Methyl red: pH <4.8 to be red
Methyl orange: pH <3.2 to be red
So the pH of the solution is pH<3.2
The drink is NOT water!
When a candle is lit, the lighter provides the _________________.
activation energy
Only one chance!
Which of the following has the largest atomic radius?
1) Li+
2) Rb+
3) K+
4) Na+
2) Rb+
atomic radius increases going down a group and decreases going left to right across a period
The Lewis dot diagram of MgCl2

The name of H2SO3
What is:
hydrogen sulfite --> sulfurous acid
(Recall for polyatomic acids: I ATE an acid and it was ICky! I only bITE into things that are deliciOUS)
If polyatomic acid ends in - ate --> - ic acid
If polyatomic acid ends in - ite --> - ous acid
1) Identify the type of reaction
2) Predict the products
3) Balance the equation
__C3H8 + __O2 -->
Combustion
C3H8 + 5O2 --> 3CO2 + 4H2O
In an energy diagram, the products have less energy than the reactants. Draw out this energy diagram.
Exothermic
The only metal in liquid state at room temperature
What is mercury
The Lewis dot diagram of SiO2 and number of lone pairs
![]()
Lone pairs = 4
The name for CuSO4 * 5H2O
What is:
Copper (II) sulfate pentahydrate
Complete and balance the reaction:
zinc hydroxide and nitric acid
Zn(OH)2 + 2HNO3 --> Zn(NO3)2 + 2H2O
Come up with a balanced decomposition equation for water, with an endothermic heat term in the equation.
2H2O + heat --> 2H2 +O2
