This is the center of an atom:
nucleus
How many valence electrons does Hydrogen have?
1
Correctly draw a Lewis Dot Structure for a molecule of H2O:


What types of elements form ionic bonds?
Metals and nonmetals.
What do you call a force that holds atoms together in a compound?
A chemical bond
These two particles make up the center of an atom:
protons & neutrons
How many valence electrons does Lithium have?
1
In a covalent bond, how are electrons involved?
They are shared between two atoms.
In an ionic bond, what happens to the electrons?
Electrons are transferred from one atom to another.
What does the suffix, "-ide" mean?
indicates a negative ion or anion, specifically one that is composed of a single atom
This lightweight particle moves around the center of an atom:
electron
How many valence electrons does Calcium have?
2
True or False: Water is made with covalent bonds.
True
What type of charge does an atom have after losing an electron and what is the name of the ion?
positive; cation
Which bond type forms gases or liquids like carbon dioxide and water?
covalent bonds
This philosopher is credited with coining the term "atomos":
Democritus
How many valence electrons does Oxygen have?
6
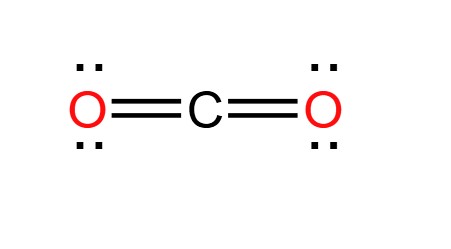
Draw a Lewis Dot Structure for a molecule of carbon dioxide:
Write down the ions for MgO:
[M+2] [O]-2
What does an atom want when it forms a bond?
It wants a full valence shell (8 valence electrons)
In a neutral atom with an atomic number of 54, what would be its number of protons and electrons?
54 protons; 54 electrons
How many more valence electrons does Xenon need in order to become stable?
0; it already has a full valence shell.
Draw the Lewis Dot structure for a Nitrogen compound

Write down the product (with the charges and amounts for each atom) for the following reactant: Ca +Cl. Then, write down the name of the compound.
CaCl₂; calcium chloride
True or False: All atoms want 8 electrons in their outer shell.