The force of attraction that holds two atoms together is a ____. (crystal lattice or chemical bond)
What is a chemical bond?
Electrons free to move throughout a material are associated with a(n) ____. (ionic bond or metallic bond)
What is a metallic bond?
A chemical reaction that gives off light is called ____. (exothermic or endothermic)
What is exothermic?
A biochemical composed of amino acids is a____ . (lipid or protein)
What is a protein?
Charged particles that form when atoms transfer electrons are ____. (molecules or ions)
What are ions?
Shared electrons are associated with a ____. (covalent bond or metallic bond)
What is a covalent bond?
A chemical reaction that forms one compound from two or more substances is called a ____. (synthesis reaction or decomposition reaction)
What is a synthesis reaction?
What type of compound would you use to neutralize a solution of potassium hydroxide?
What is an acid?
This type of element tends to lose electrons when it forms bonds. (metal/nonmetal/metalloid/noble gas)
What is a metal?
The type of bond two oxygen atoms will form.
What is a covalent bond?
The type of reaction in which ions in two compounds switch places.
What is a double-replacement reaction?
A source of energy for living things can be found in ___. (nucleic acids or carbohydrates)
What are carbohydrates?
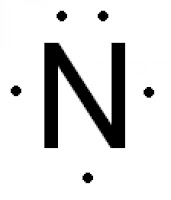
The electron dot diagram for Nitrogen.
The type of bond potassium (K) and fluorine (F) will form.
What is an ionic bond?
Balance the following equation.
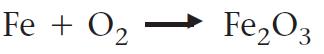
4Fe + 3O2 -> 2Fe2O3
An increase in the concentration of hydronium ions in solution _____ the pH.
What is lowers?
The number of electrons Calcium must gain or lose to have 8 valence electrons. Then calculate the charge of the ion that would form.
What is 2? What is 2+?
Explain why an iron ion is attracted to a sulfide ion but not to a zinc ion.
Zinc loses electrons to get 8 valence electrons, giving it a positive charge. Sulfur gains electrons to get 8 valence electrons, giving it a negative charge. The positive iron ion is attracted to the negative sulfur ion.
Which letter represents the activation energy?
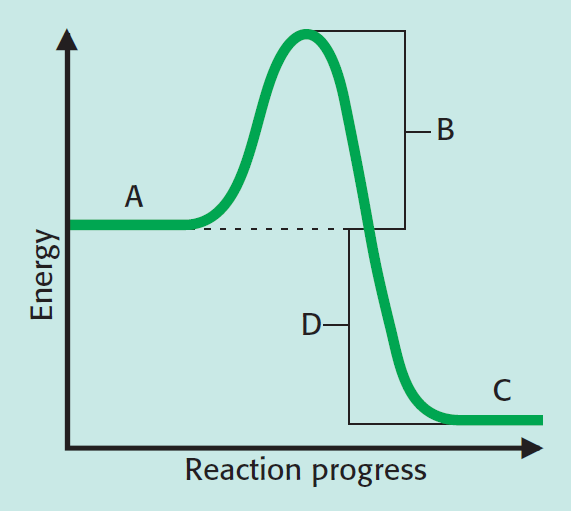
B
Fish give off the base ammonia, NH3, as waste. How does the release of ammonia affect the pH of the water in the aquarium? What can be done to correct the problem?
Increases pH / add acid