What is the chemical bond formed between two non-metals?
What is covalent/molecular?
What type of equation is this? Predict the products and balance
NH3 + HCl ----> ?
Synthesis, NH4Cl
What effect does surface area have on a chemical reaction? Provide an example.
It speeds up the rate of chemical reaction. Marble chips vs marble powder.
What is Avogadro's number?
6.022 x 1023
What ions are present in acids and what are present in bases?
H+ ; acids, OH- ; bases
What is the chemical formula for aluminum sulfide?
Al2S3
Predict the products and balance the equation:
C2H4 + 3O2 ----> ?
2CO2 + 2H2O
Why do catalysts not appear in the chemical equation?
They are not an end product in the equation and do not change throughout the chemical reaction.
How many moles are in 15 grams of lithium?
2.16 moles
What is the difference between acids in bases in terms of H+ ions?
acids produce them while bases accept them
How many of each element are needed when phosphorous and chlorine bond?
1 P and 3 chlorine
What type of reaction is this? What are the products? Balance the equation.
RBNO3 + BeF2
Does not happen because all products are aq.
What are the five factors affecting chemical reactions?
size, concentration, temperature, surface area, and the presence of a catalyst
What are the molar masses of each side of the equation?
C2H4 + 3O2 ---> 2CO2 + 2H2O
124.052, 124.052
What substance would turn green with bromothymol blue and stay clear in phenolphthalein?
Water (any neutral compound)
Find the formula of Iron (III) Carbonate
Fe2(CO3)3
What is the difference between these two equations and explain the reason why:
CH4 + 2O2 -----> CO2 + 2H2O
4CH4 +5O2 -----> 2CO + 8H20 + 2C
The first one is complete combustion while the second one is incomplete combustion. The reason for this is because there was not enough oxygen to react with the fuel in order to produce carbon dioxide and water.
What is the difference between bond energies in endothermic and exothermic reactions?
In exothermic reactions, the energy that is released when new bonds form is greater than the energy needed to break them.
In endothermic reactions, the energy needed to break them is greater than the energy released.
It has been estimated that 93% of all atoms in the entire universe are hydrogen and that the vast majority of those remaining are helium. Based on only these two elements, estimate the mass percentage composition of the universe.
Mass percentage of Hydrogen = 76.86%
Mass percentage of Helium = 23.14%.
What are the products of a neutralization reaction?
Water and an ionic compound
What element is this?
2, 8, 18, 18, 1
Silver (Ag)
Sodium metal reacts with hydrochloric acid, HCl, and produces hydrogen gas as one of the products. What is the full equation (balanced and products)?
2Na + 2HCl ---> 2NaCl + H2
Looking at the graph on the right, what does this show? Explain why.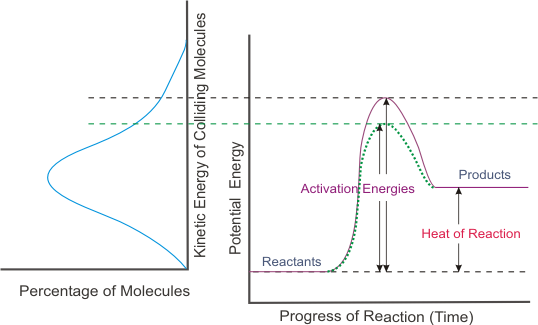
An endothermic reaction because the products are at a greater energy level than the reactants
How many moles of Na+ ions are present in 20 mL of 0.40M Na3PO4?
0.024
What happens when a solution of an acid is mixed with a solution of a base in a test tube (two)?
1. The temperature of the solution increases
2. Salt formation takes place