The center of the atom.
What is the nucleus?
The atomic number of an element refers to this number of subatomic particles.
What is a proton?
The shared valence electrons between two atoms.
What is a chemical bond?
Anything that has mass and occupies space.
What is matter?
What is sublimation?
A sub-atomic particle that has no electrical charge.
What is a neutron?
The electrons that are on the outer orbit of an atom.
What are valence electrons?
The process that rearranges the molecular structure of a substance.
What is a chemical reaction?
A characteristic of an object that can be measured or observed.
What is a property?
This must be added or taken away for matter to change form.
What is energy?
The home of the electrons.
What is an orbital?
What is 7?
The number of electrons almost every atom wants to have in the outer orbit.
What is 8?
This tiny particle is the smallest unit of matter.
What is an atom?
This creamy substance is an example of all three states of matter in one.
What is ice cream?
This subatomic particle can be shared with another atom.
What is an electron?
According to this picture, Oxygen has this number of electrons and what other subatomic particle?

What are protons?
These two elements are the exception to the rule of octet.
What are Hydrogen and Helium?
A group of atoms bonded together
What is a molecule?
The process in which liquid becomes a gas on the surface or the liquid.
What is evaporation?
Adding the number of protons and neutrons gives you this atomic measurement.
What is the Atomic Mass?
The element represented by the atomic symbol Au.
What is Gold?
This Lewis Dot Diagram shows the chemical bonding for which molecule?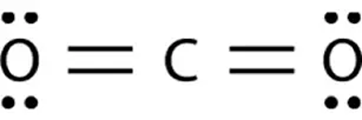
What is Carbon Dioxide?
A simplified way to represent the valence electrons for an atom.
What is a Lewis Dot Structure?
A liquid like substance that acts like a solid when under pressure.
What is a Non-Newtonian liquid?