This is our name for all the stuff that the Universe is made of.
 What is matter?
What is matter?
The periodic table is a chart that organizes these basic ingredients of matter.
What are elements?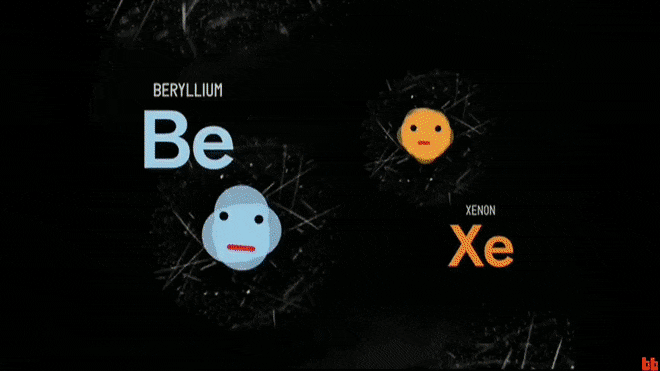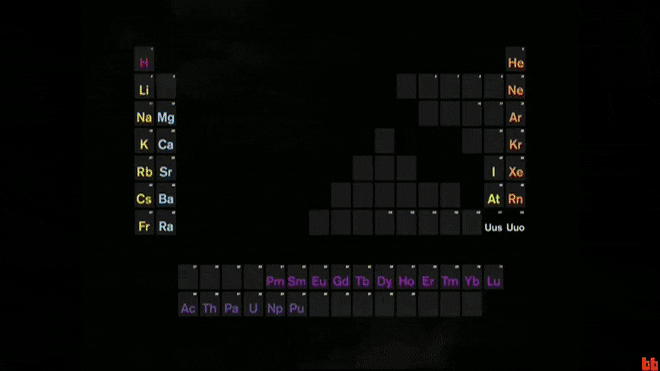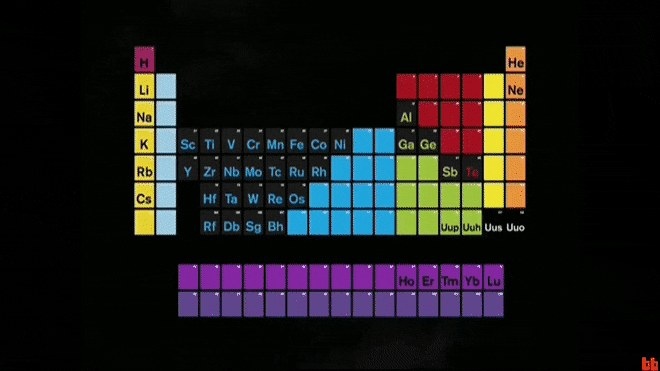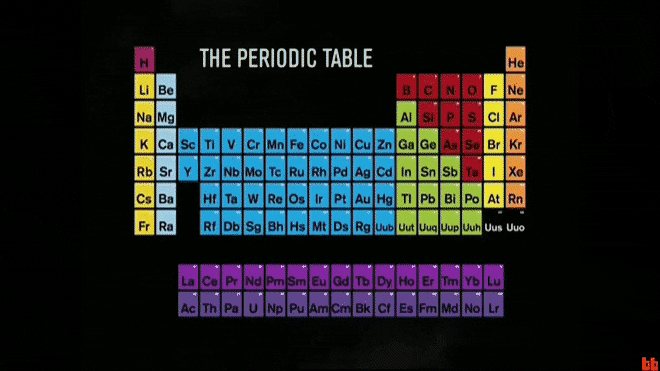
A change in matter that can usually be reversed and does not create a new substance.
What is a physical change?
Stored energy
What is potential?
This type of wave needs no matter to travel through.
What is an EM wave?
This is our name for the center of an atom.
What is the nucleus?
Found in group 18, these elements do not react with other elements.
What are noble gases?
A change in matter that usually can't be reversed and results in a new substance being created.
What is a chemical change? 
Energy being used or in motion
What is kinetic energy?
What are gamma waves?
A particle that is made from 2 or more atoms chemically bonded together.
What is a molecule?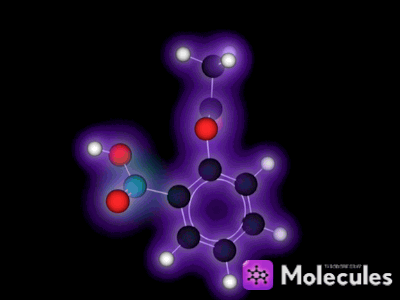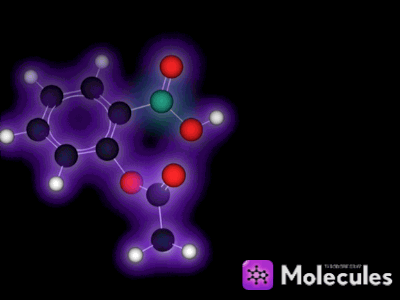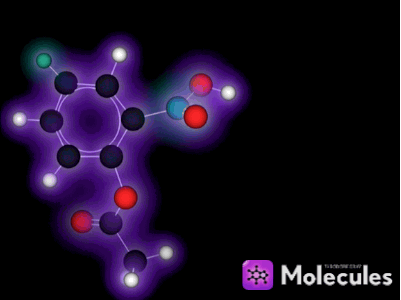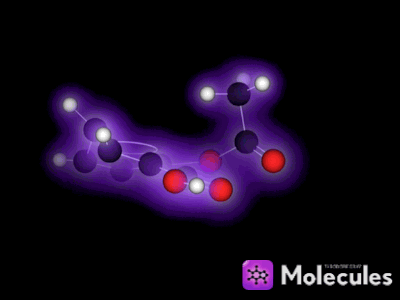
The largest family group on the periodic table, these elements are malleable and conduct electricity.
What are metals?
This is a sign that a chemical change has occurred (any one will do)
Light or heat, gas produced, new substance formed, an odor, color change etc.
All EM radiation is this type of energy.
What is kinetic?
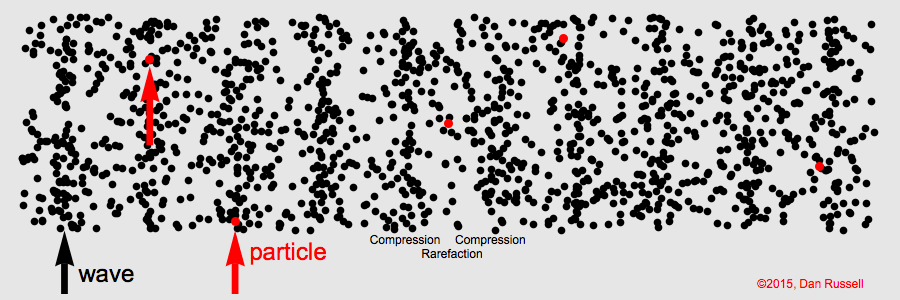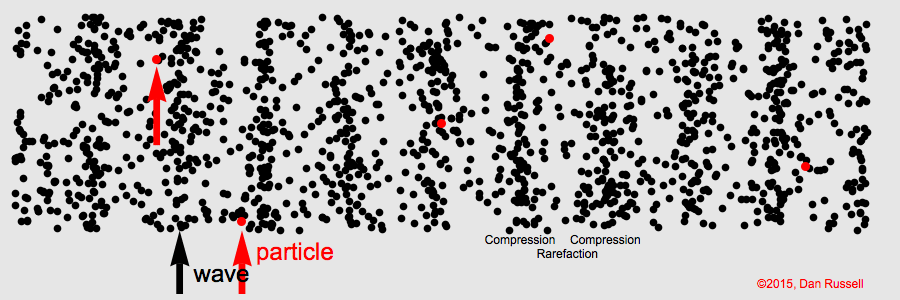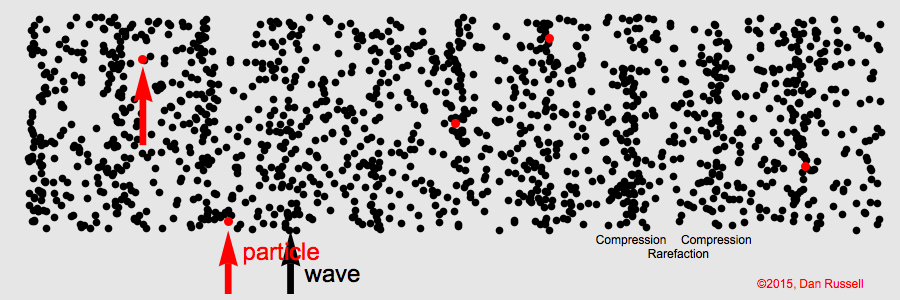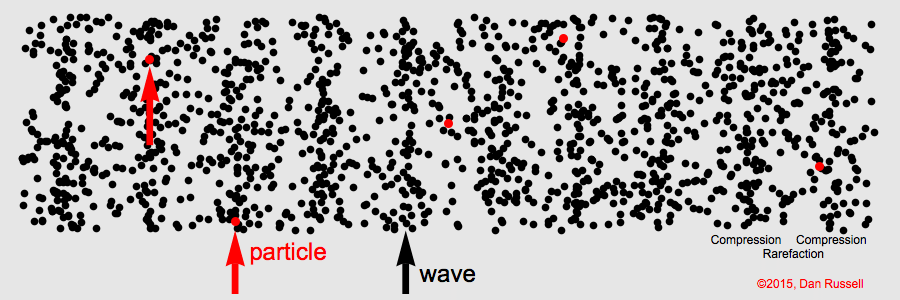
The term for the matter that a mechanical wave needs to travel.
What is "medium"
An atom that has a positive or negative charge.
What is an ion?
This is the only known element with no neutrons in its nucleus.
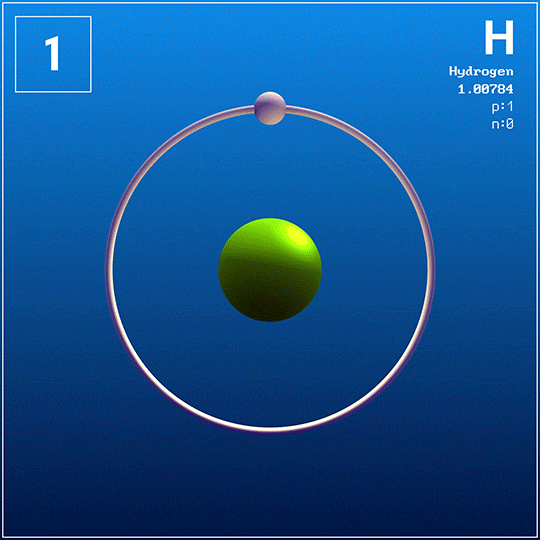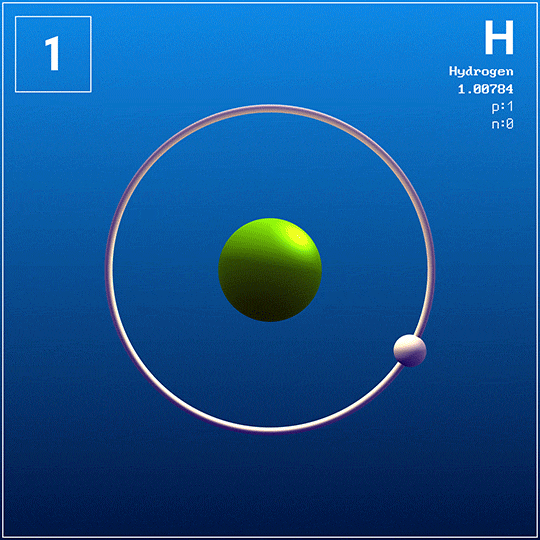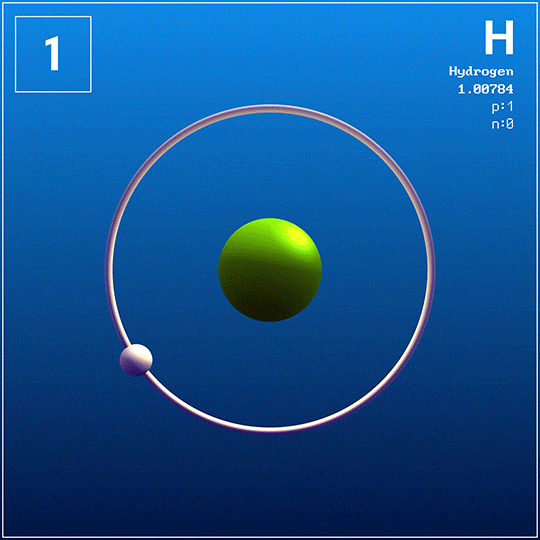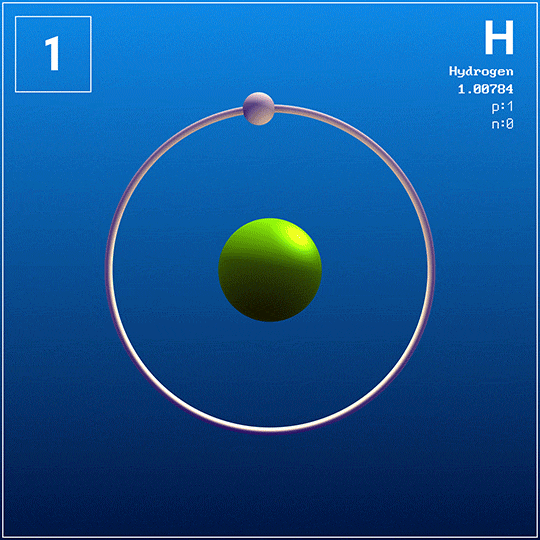 What is hydrogen?
What is hydrogen?
This is an example of a physical change in matter.
(Any accurate example will do) What is breaking, tearing, melting, freezing, cutting etc...
A flashlight changes this type of energy into light energy.
What is chemical (or electrical potential )
)
The term for the height of a wave or how much energy it carries.
What is amplitude?
This is the positively charged particle in the nucleus of an atom.
What is a proton?
Our word for elements that have the same number of protons but different numbers of neutrons in their nucleus.
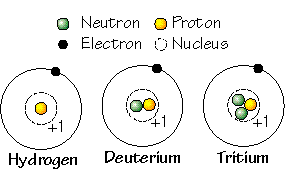 What is isotopes?
What is isotopes?
This is our term for the new substance(s) created after a chemical change.
What is "product(s)"?
This type of energy is dependent upon an object's position.
What is Gravitational Potential Energy?
The area of a longitudinal wave where particles are spread thinnest.
What is a rarefaction?