Combustion Reactions do what?
What is Combining?
A bond between metals
What is Metallic Bonds?
The dot in a Lewis Dot Diagram is...
What is An Electron?
Elements number in H2O?
What is 3? (2 Hydrogen 1 Oxygen)
A single element and a molecule are the same
The difference between single and double replacement reactions
What is The Amount of Elements Replaced
Non-metals form blank bond
What is Covalent Bonds?
One covalent bond between atoms
What is a Sigma Bond?

Element number in: C4Cl
What is 5? (4 Carbon and 1 Chlorine)
Natural Polymer Definition
What is a polymer that occurs naturally.
Force of attraction that holds positive and negative ions together
What is an Ionic Bond?
The difference between polar and nonpolar Covalent Bonds
What is Polar Covalent Bonds sharing atoms unequally, and nonpolar covalent bonds sharing atoms equally
The Lewis Dot Diagram for Lead
Pb with four dots around it
Magnesium needs to lose or gain electrons to have a full outside shell
What is to Lose?
Most decomposition reactions require blank to occur
What is Energy (Heat, Light, Electricity)
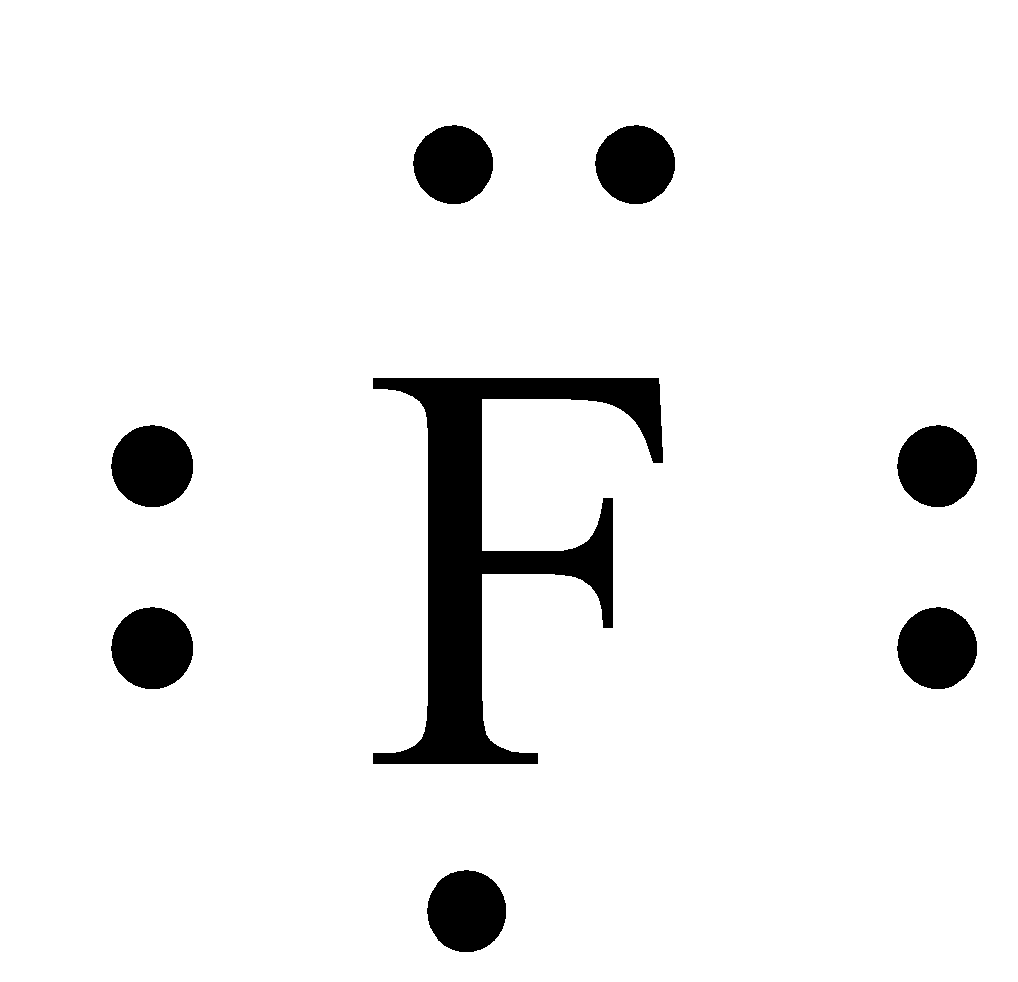
The Lewis Dot Diagram of Fluorine
Types of polymers we learned about
What is Natural and Synthetic polymers?
Within a Single Replacement Reaction a rule when replacing is...
What is a non-metal must be replaced with another non-metal?
Balance: CH4 + O2 --> CO2 + H2O
CH4 + 3O2 --> 2CO2 + 2H2O
The definition of both Exothermic and Endothermic Reactions
What is a chemical reaction in which less energy is needed to break bonds in the reactants than is released when new bonds form in the products? What is chemical reactions in which the reactants absorb heat energy from the surroundings to form products?