& lab safety
Define density.
Density is mass per unit volume.
Define compound.
A substance that consists of two or more elements chemically combined together.
What is filtration?
A method used to separate insoluble solids from liquids.
State the meaning of the hazard symbol.
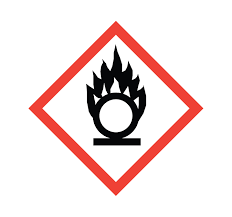
Oxidising substances burn in the absence of air, and can intensify fires in combustible materials. Should be ket away from ignition sources.
Tungsten is a suitable material for making the filament in a light bulb. What property does it have?
high melting point
Which model best represents a compound?
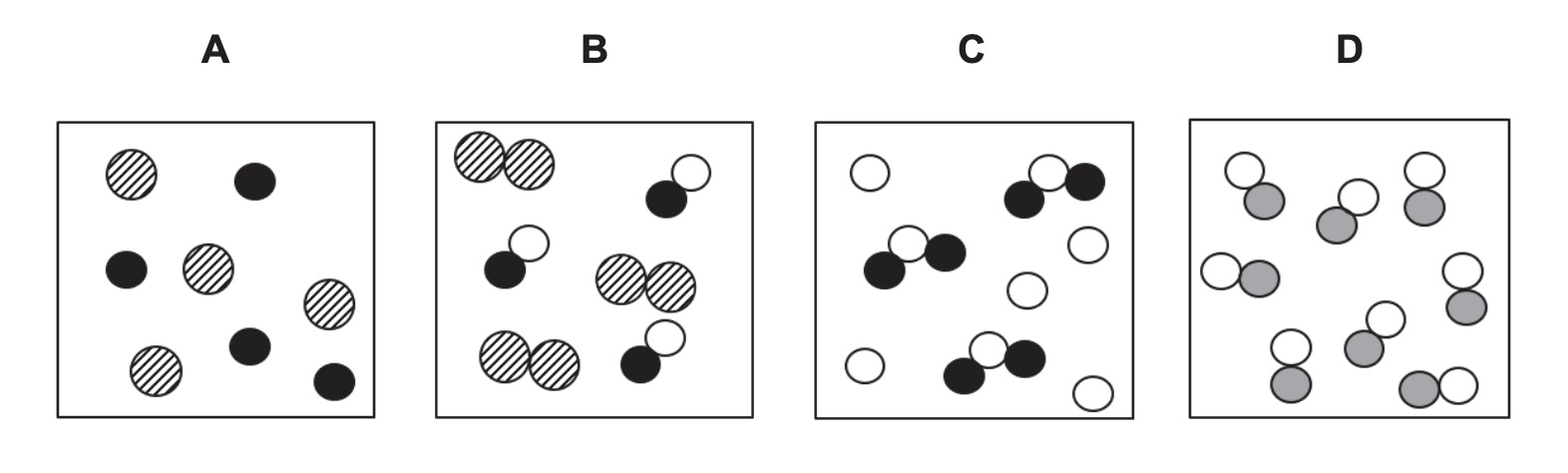
D
A sample of table salt was contaminated with barium sulfate powder and iron filings.
Barium sulfate is insoluble in water.
State, in the correct sequence, the separation techniques used to separate and collect dried sample of each substance.
magnetic attraction --> dissolve in water --> filtration --> evaporation
The scientific method usually involves the following steps:
identify a problem --> X --> conduct experiment --> Y --> make conclusion
Which steps do X and Y represent?
X - make a hypothesis
Y - collect experimental data
The tip of the drill bit is made of .................... because it is a very ................. material.
diamond, hard
State the factors that affect solubility.
(i) type of solute
(ii) type of solvent
(iii) temperature
Drinking water can be obtained from treatment of used muddy water.
The figure below shows a filter tank used in the first stage of the purification process.
(a) Explain how the filter is used to purify water. (b) Suggest one improvement that can be made to the design of the filter tank.
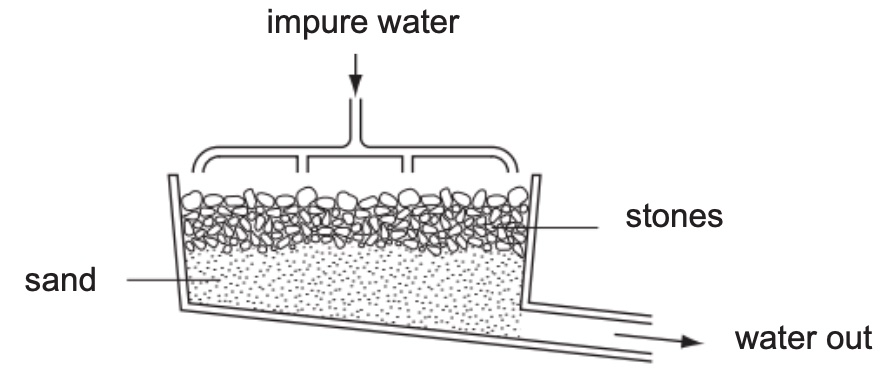
(a) The filter does not allow particles larger than the spaces in between stone and sand to pass through, leaving the impurities as residue and water as filtrate.
(b) Add extra layers of gravels or activated carbon.
Caleb carried out an experiment to find out the relationship between the mass of a ball and how fast it falls from a height. The results are shown in the table.
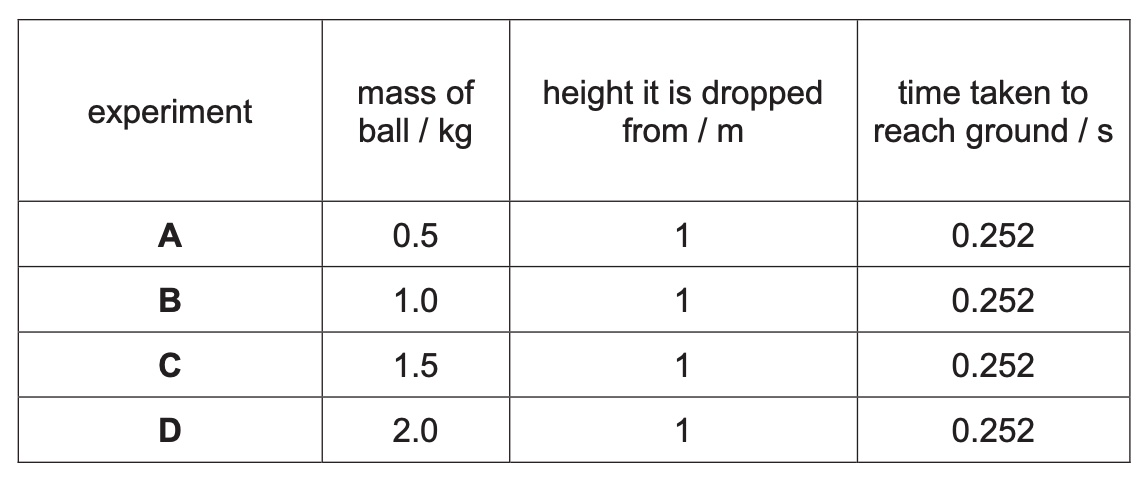 (a) State the dependent variable in the experiment.
(a) State the dependent variable in the experiment.
(b) Suggest a conclusion for this experiment.
(a) time taken to reach the ground
(b) Mass of the ball does not affect the time taken to reach the ground
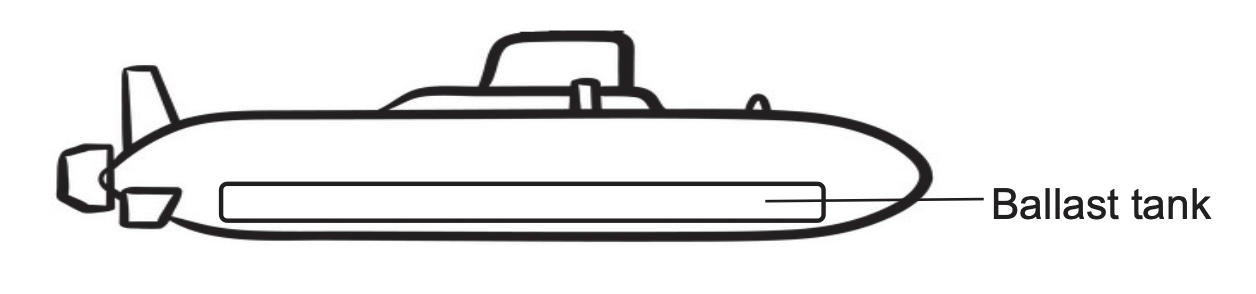 To make the submarine float, the ballast tanks are filled with air. To make it sink, the ballast tanks are filled with water.
To make the submarine float, the ballast tanks are filled with air. To make it sink, the ballast tanks are filled with water.
When the ballast tanks are filled with water, how do the submarine’s total mass, volume and density change?
mass increases,
volume remains the same,
density increases.
List all the elements and the number of atoms present in CH3COOH.
Carbon, Hydrogen, Oxygen
8 atoms
(a) Name this separation technique.
(b) Which ink contains only one dye?
(c) Explain the results for ink B.
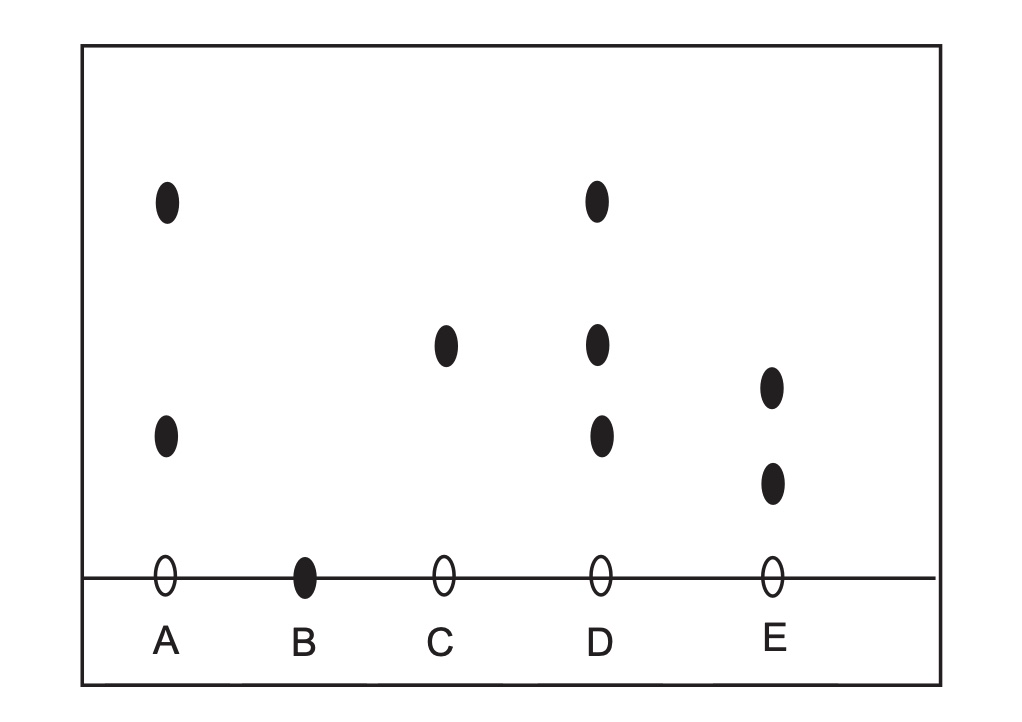
(a) paper chromatography
(b) ink C
(c) Ink B is not soluble in the solvent.
Jordan noticed that after several rounds of washing, some of his T-shirts shrank in size. He then conducted a scientific investigation.
I. Jordan measured the size of the T-shirts after the wash and recorded them in a table.
II. Jordan concluded that cotton results in greater shrinkage.
III. Jordan chose two T-shirts, made up of cotton and polyester respectively, and washed them under the same conditions.
IV. Jordan predicted that the materials of the T-shirts affect whether they would shrink in size after washing.
Arrange the steps above in the correct sequence.
IV, III, I, II
Describe the method used to find the density of an irregular object (e.g. stone). State clearly the apparatus used.
Measure the mass of the irregular object using a mass balance.
Fill up a 100 ml measuring cylinder with 50ml of water. Place the object into the cylinder and measure the increase in volume. The difference in volume would be the volume of the object.
Calculate density by dividing mass by volume.
Tim wanted to fully dissolve a block of table salt in water at room temperature, in the shortest time possible. State three actions he could perform to increase the rate of dissolving of the table salt.
1) The block of table salt can be crushed / made into a powder / broken up into smaller pieces before it is added to water;
2) Water can be heated up so that it is of a higher temperature;
3) mixture of salt and water can be stirred
The diagram below which shows part of an apparatus used in a separation technique.
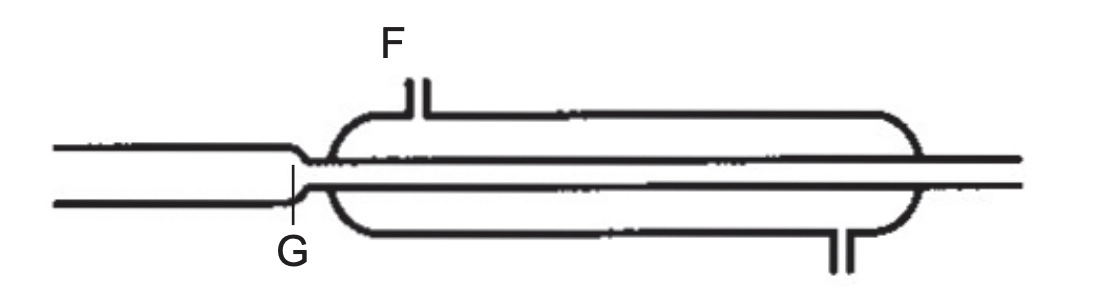 (a) What is this apparatus and in which separation technique is it used in?
(a) What is this apparatus and in which separation technique is it used in?
(b) What happens at F and G?
(a) condenser; distillation
(b) F is the outlet for water to move out
(c) G is the point before hot water vapour enters the condenser.
When the air hole of the Bunsen burner is opened, a certain type of flame is obtained.
(a) Name this type of flame.
(b) Describe the colour of this flame.
(c) Suggest how this type of flame is useful.
(a) non-luminous flame
(b) light blue
(c) It is used for heating as it is very hot and does not produce much soot.