Anything that has mass and occupies space
What is matter?
Have a definite volume but not a definite shape.
What are liquids?
Consists of at least two different substances, that is, it contains at least two types of particles
What is a mixture?
A homogeneous mixture in which it is impossible to distinguish its constituent parts, even under a magnifying instrument
What is a solution?
The maximum amount of solute that can be dissolved in a given amount of solvent.
What is solubility?
The concentration when 10g of sugar are dissolved in 2L of water.
What is 5g/L?
A solution that contains less than the maximum amount of solute.
What is unsaturated?
Rico needs to have 250 mL of a saline solution with a concentration of 15 g/L. How many grams of salt does he need?
What is 3.75 g?
A scientific model based on the idea that matter is made up of small particles
What is the particle model?
Occupy all of the space they are given. Do not have a definite shape or volume.
What are gases?
Where we can find all of the atoms known to man.
What is the periodic table?
Consists of one substance, that is, it contains only one type of particle
What is a pure substance?
A laboratory technique that involves decreasing the concentration of a solution by adding solvent.
What is dilution?
The concentration (g/L) when 60g of salt are dissolved in 400 mL of water.
What is 150 g/L?
A solution that contains the maximum amount of solute.
What is saturated?
The concentration, in % m/V, of 400 ml of solution containing 60 g of solute
What is 15% m/V?
The three phases of matter.
What are solid, liquid, and gas?
The smallest particle of matter. It cannot be divided by chemical means.
What is an atom?
Made up of at least two substances that cannot be distinguished with the naked eye
What is a homogeneous mixture?
A homogeneous mixture in which at least two different substances can be distinguished under a magnifying instrument
What is a colloid?
Corresponds to the quantity of dissolved solute in a given quantity solvent.
What is the concentration?
The amount of solute in 150 mL of a 75 g/L solution.
What is 11.25g?
A solution that contains more than the maximum amount of solute.
What is supersaturated?
The volume of a solution containing 450 g of solute if the concentration of the solution is 300 g/L.
What is 1.5 L?
Very organized. The particles can only vibrate in one spot. Have a definite shape and volume.
What are solids?
A group of two or more atoms held together by chemical bonds
What is a molecule?
Made up of at least two substances that can be distinguished by the naked eye.
What is a heterogeneous mixture?
The substance that dissolves in another substance (solvent).
What is a solute?
Called the universal solvent. It is a very common substance.
What is water?
The four characteristics that can be used to identify a gas.
What are: density, reaction to a glowing splint, reaction to a lighted splint, reaction to limewater and solubility?
This forms when there is surplus solute.
What is a precipitate?
The density of a solid with a mass of 46.4 g and a volume of 2.4 L.
What is 19.3 g/L
Identify this state. No it is not Alaska!
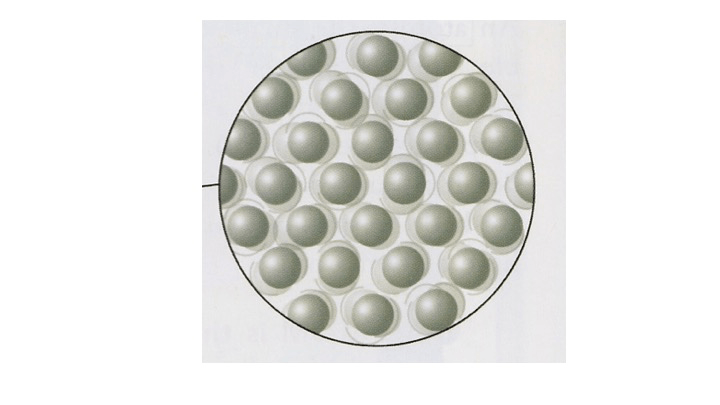
What is a solid?
Identify the state:
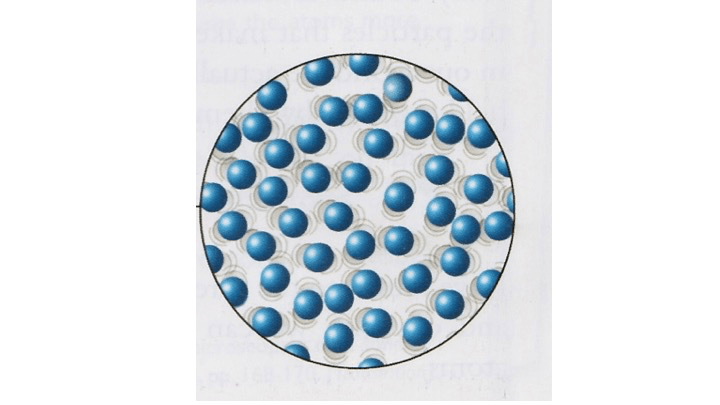
What is a liquid?
Identify the state:
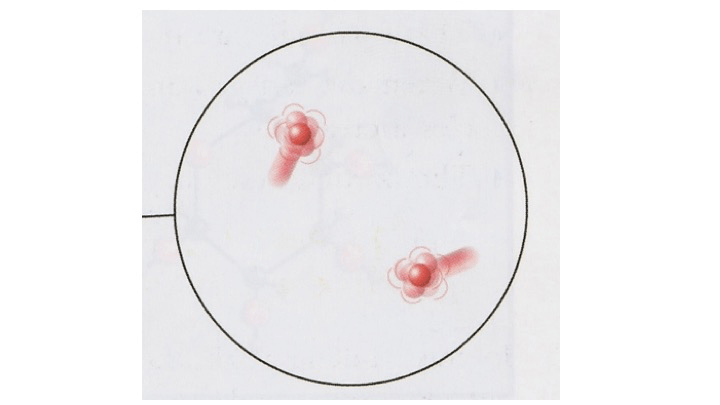
What is a gas?
The substance that dissolves a solute.
What is a solvent?
A mixture of atoms in which the primary constituent is a metal.
What is an alloy?
When exposed to an open flame, this gas explodes.
What is hydrogen?
Substances are not soluble in water.
What are hydrophobic substances?
The mass of gas in a 20-L tank if the density of propane gas is 0.001 83 g/ml at 20 °C and normal atmospheric pressure.
What is 36.6 g?
This gas causes limewater to turn cloudy.
What is carbon dioxide?
Has a pH less than 7.
What is an acid?
Milk, mayonnaise and blood are examples of this type of mixture.
What is a colloid?
This gas will cause a glowing splint to relite. The gas that allows for combustion.
What is oxygen?
Has a pH of 7.
What is a neutral solution?
Substances that are soluble in oil are lipophilic. Substances that soluble in water are an example of this type of solvent.
What are hydrophilic?
A common liquid with a density of 1 g/mL and a boiling point of 100 Celcius.
What is water?
Has a pH greater than 7.
What is a base?