What does the graduated cylinder measure?
volume
Is this molecule a polar or non-polar covalent bond?
polar covalent bond
Chemical or physical change:
A flask filled with liquid water is heated over a burner to a temperature of 100°C and turns into gas.
Physical
What is this separation technique called? 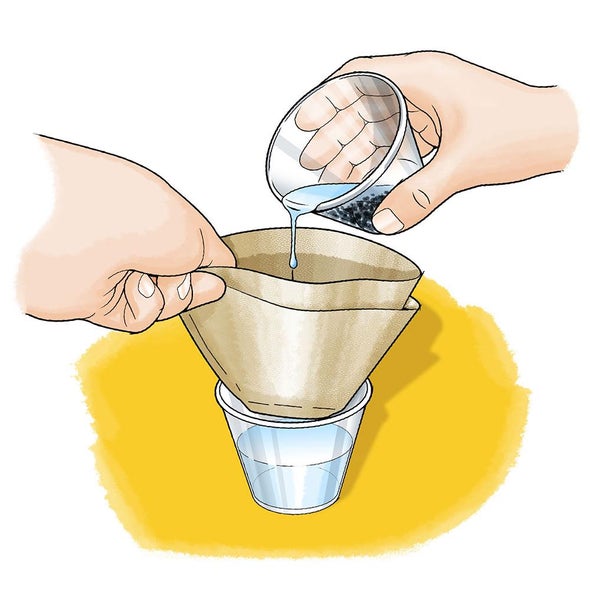
filtration
What element is in group 3 and period 4?
Scandium (Sc)
A metal cube has sides measuring 5 cm in length and a mass of 265. What is the density? You must include the unit of measurement!
2.12 g/cm3
Which element is the anion?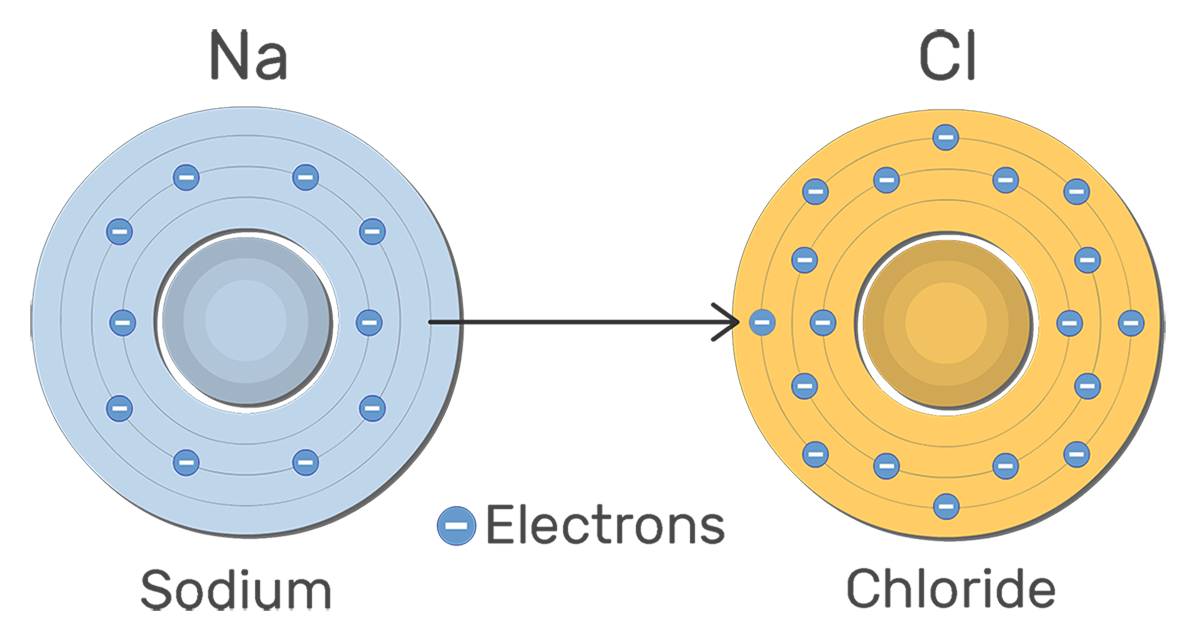
Chlorine (Cl)
Chemical
KCl and NaCl are the same solubility at what temperature?
30°C
True or False: Phosphorous (P) and arsenic (As) have similar chemical properties.
True!
What happened to the total mass of a sheet of paper before and after it was cut up to make this paper snowflake?

The mass of the paper was greater before it was all cut up into a snowflake because mass was REMOVED!
_____ can be described as a(n) _______ due to its positive charge.

Sodium (Na); cation
Is this a chemical or physical change?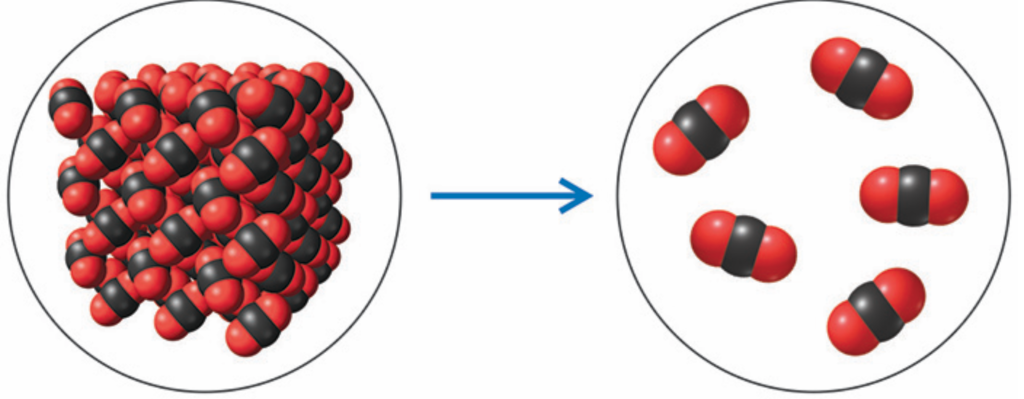
Physical
70g of KCl is added into a beaker of 100g of water that is heated to a temperature of 40°C. Is the solution saturated, unsaturated, or supersaturated?
Supersaturated.
Create a Bohr model for fluorine (F)

What is the density of an object with a volume of 16mL and a mass of 48g? You must include the units of measurement!
3 g/mL
Oxygen gains two hydrogen atoms. What is oxygen's net charge?

-2
Is this a chemical or physical reaction?

Chemical
100g of water is heated to a temperature of 70°C. How many grams of KClO3 should be added to result in a saturated solution?
30g
Name the 3 subatomic particles and their charges.
Protons = positive
Neutrons = neutral
Electrons = negtaive
True/False: The total mass of the substances before they were combined is greater than the total mass of the substances after they were combined.
False! The total mass stays the same.
What is the difference between ionic and covalent bonds?
Ionic bonds: electrons are given and gained.
Covalent bonds: electrons are shared.
Describe how a chemical reaction occurs.
When bonds are broken and/or new bonds are created. This results in an entirely new substance.
A beaker of 100g of water is heated to a temperature of 60°C. How many grams of KClO3 could be added to the beaker to result in an unsaturated solution? (Give any amount that would result in an unsaturated solution!)
0-19g of KClO3
What element does the Bohr model represent? 
Sodium (Na)