The number of electrons in Chlorine
What is 17
Atoms have no electric charge because they have __________.
Have an equal number of protons and electrons
Electrons in ionic bonds behave in this manner
Transferred from one atom to another.
Which symbols represent atoms that are isotopes?
C-14 and N-14
I-132 and I-132
O-16 and O-18
Rn-222 and Ra -222
O-16 and O-18
What is the charge of any element that gained 2 electrons
-2
One-Third of the periodic table is considered?
Non-metals.
(Metals are 2/3rds of it)
A subatomic particle that has a positive charge is called a(n)
What is a proton
In non Polar bonds, electrons do this.
shared equally by two atoms.
What is the most electronegative element on the periodic table?
Fluorine
Anions have what charge?
negative.
(Cations are positive)
Mendeleev organized his periodic table by what similar item?
Atomic masses
What is the definition of an isotope?
atoms of the same element with a different number of neutrons.
Naming the atom with atomos.
1s22s22p63s23p5 is the electron configuration for which element
This is Chlorine.
If an element has an atomic number of 17, what does this mean?
it has 17 protons in its nucleus
The atom's of nucleus contains these 2 subatomic particles.
Protons and neutrons.
What is the number of protons of this element?
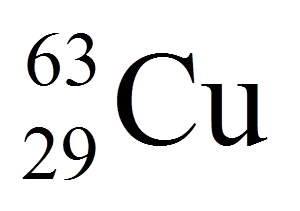
29
A sample of element X contains 90% X-25, 8.0% X-27, and 2.0% X-28 atoms. The average atomic mass will be closest to which value?
What is 25.
The key words are closest to!! I do NOT want you to calculate this, I want you to critically think about percentages.
An ionic compound is made up of what two types of elements?
A Metal and a Non-metal
The number of neutrons in a neutral atom with a mass number of 86 and 33 electrons is
86-33 = 53
This column is called the Halogens
Column 17.
How many protons does sodium have?
11
This is the picture of a nucleus of what element?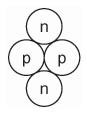
Helium
Covalent bonds have the characteristics of:
Low melting points.
Sugar melts! Also a lot of gases are covalent.
Ionic bonds are most know for which characteristic?
high melting points.
Think of how hard it is to melt salt.
What is section 4
Transition metals.
1. metals
2. inner transition metals
3. non-metals
Overall, this group has the lowest ionization energy.
Alkali Metals
This represents what type of bond?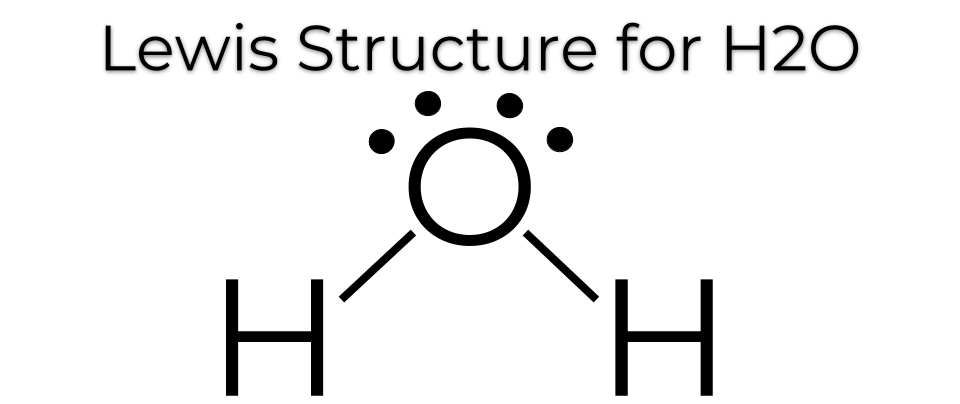
Polar Covalent
The following electron dot diagram represents a molecule that has what type of bond:
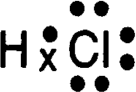
Polar Covalent.
Polar- Uneven sharing
Non-Polar- Evenly sharing.
Metallic bonds are _________ in water.
Not Soluble in water