density is the relationship between____
Mass and Volume
what are the forces that hold together the particles in this picture:
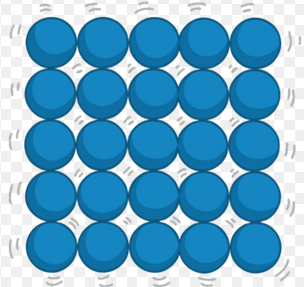
Inter- and Intra- molecular forces
where is the energy coming from and going to in this picture:
Girl to air
Girl to Ice cream
What direction do fluids move when heated?
They rise/ go up
What particles are in an atom?
Protons, Neutrons, Electrons
the density of oil is 0.88 g/ml, and the density of alcohol is 0.3 g/ml.
describe how the layers would look when placed in a graduated cylinder and why.

Alcohol on top, Oil on bottoms, because alcohol is less dense than oil
the particles for the image shown are likely what state of matter? why?
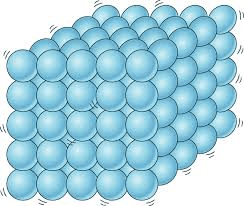
Solid, because they are packed closely and not moving much.
this mini-fridge is what type of system? why?

Open system because energy and matter can move in and out freely.
Why do fluids rise when heated?
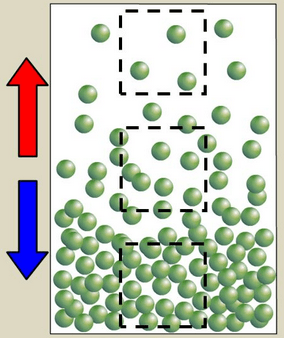
The particles in a fluid separate and become less dense, allowing them to float above other fluids.
This is a bohr model of what element? How do you know?
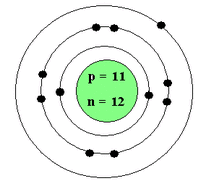
Sodium because the number of protons matches Sodium's atomic number 11.
which of the following has the highest density? why?
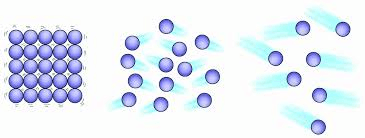
The one on the left because the particles are the most packed together.
Order the following substances from weakest to strongest. why is it that order?
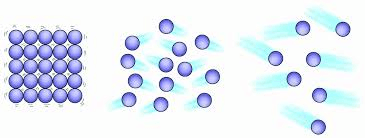
A B C
C,B,A because C has the least amount of intermolecular forces and A has the most
Montes's students do a chemical reaction and record the temperature over time. what type of reaction do they have based on the data? why?
Table:
DeltaH_"absorbed" = 1020kJ
DeltaH_"released" = 1244kJ
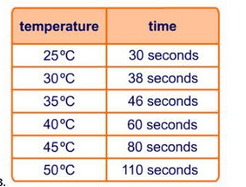
Exothermic since heat is released and increases the temperature (Kinetic Energy).
Fernando wants to cool down after his workout, where in Hawkins should he hang out? Why?
Bottom floors because cold air is dense and sink towards the bottom. Hot air will rise.
How many Valence Electrons does an atom of lead (Pb) have? How do you know?
4 Valence electrons because lead is in main group 4.
What is the density of a substance that has a mass of 32g and displaces the following volume:
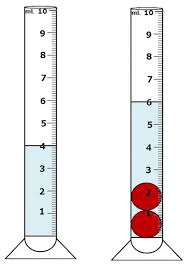
D=(Mass)/(Volume)
16g/mL
What is happening between section D and E. How do you know?
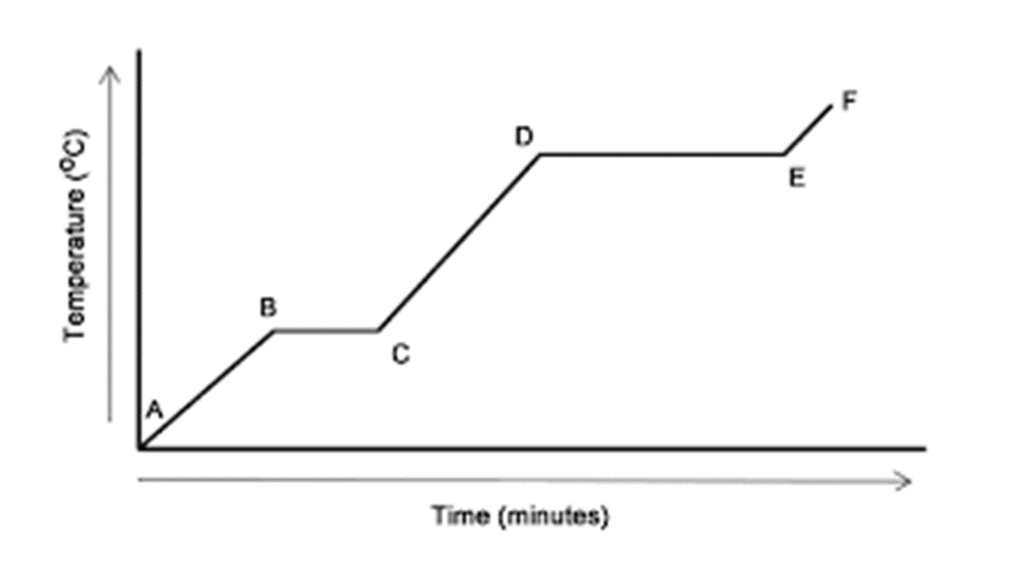
The substance is boiling because the temperature of a substance cannot rise while it is boiling.
What type of reaction does this graph show? How do you know?
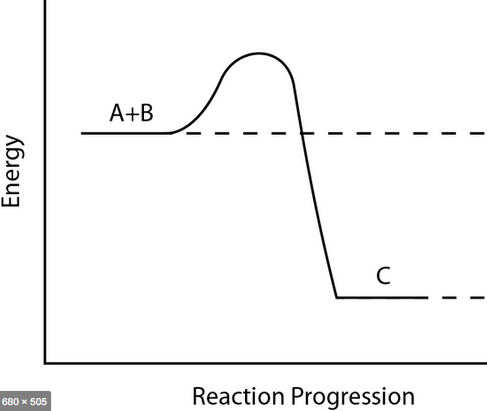
Exothermic reaction because the energy goes from high to low, meaning energy was released.
What would happen to the liquids the instant they are brought together if the yellow liquid is 80oC and the blue liquid is 15oC. Why?

Nothing because heat rises so the warmer layer will stay on top and the cooler layer will stay below.
Over time however, you would expect them to reach a similar temperature and mix.
What will the Ion symbol for Aluminum be if it completes it's octet (Emtpties or Fills it's Outside Shell)?
Al+3
What is the density of the substance in the graph?
*Bonus 100 pts if you can tell me what substance it is*
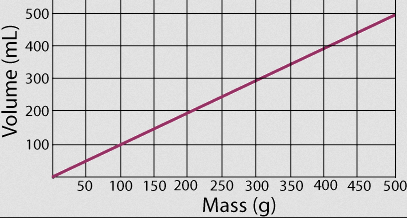
D=(Mass)/(Volume)
1.0g/mL
Which of the two substances has stronger intermolecular forces? Why?
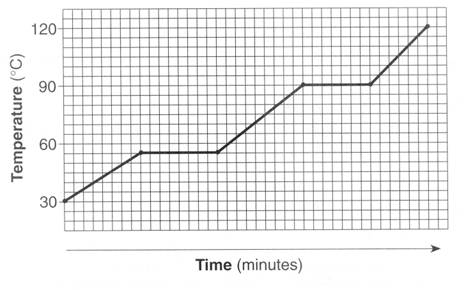
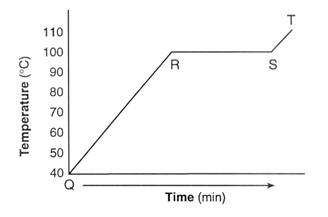
The bottom graph because the boiling point is higher, meaning it takes more energy to break the intermolecular forces holding together the particles.
(The boiling point is the portion of the graph that is flat).
How much energy would it take to change a river's temperature from 30oC to 35oC. Assume a river has 5000g of water. *Specific Heat of Water= 4.18 J/gC*
Use
q=mcDeltaT
q=5000gxx4.18J/(gC)xx5C
104,500J
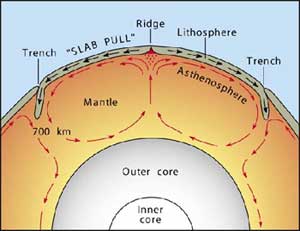
How do convection currents cause plate tectonics to move?
The earths core heats the magma under the crust causing it to rise, push the crust, and sink as it cools in a convection current.
A student finds a chunk of hard solid on the floor.
She notices it changes color as it touches the air and as she walks home it has completely turned brown.
She thought it would be a cute phone accessory but when charging her phone it touched the outlet and sparked.
Finally she realized it reacted with the plastic on her phone and melted it. So she threw it away....
What family could this element belong to? Why?
*Bonus 100pts how many Valence Electrons could it have?*
Likely a Metal because it is reactive with non-metal and conducts electricity.
