The Periodic Table
The Periodic Table
& Mixtures
of Matter
Transformations
Transfers
How many protons are in the nucleus of a gold atom?

79 protons
What are two types of pure substances?
Elements and compounds
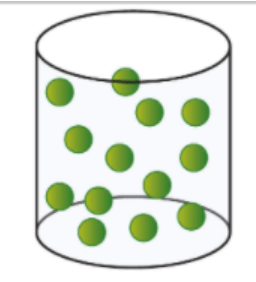
Which image depicts a gas? How do you know?
The last image is a gas. The gas phase is a state of matter where particles (atoms or molecules) are far apart, move freely and randomly, lack a fixed shape or volume, and expand to fill any container.
Describe the energy transformations that occur when a battery-powered flashlight is lit.

Chemical energy (battery) to electrical energy (wires) to radiant and thermal energy (light bulb).
Hot coffee is stirred with a spoon, the spoon gets hot due to _______________.

Conduction
What element is in period 4 group 9?

Spot the mistake!
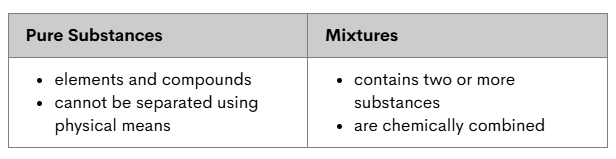
Mixtures are NOT chemically combined.
Which of the following is a chemical change: melting ice, boiling water, or rusting iron?
Rusting iron

What type of energy is stored in food before your body uses it?

Chemical potential energy
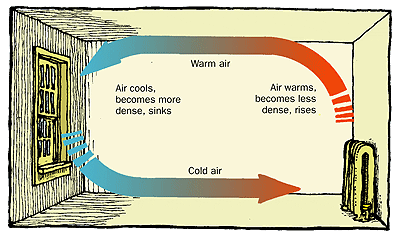
Near the ceiling of a room the air is warmer. The warm air rises because of _______________
Convection
What is the location AND charge of the subatomic particles of an atom?
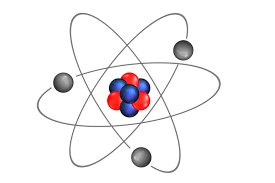
Protons are positive, they are located in the nucleus of the atom.
Electrons are negative, they are located in the electron cloud.
Neutrons are neutral, they are located in the nucleus of the atom.
This type of mixture appears uniform throughout, like lemonade.
Homogeneous mixtures
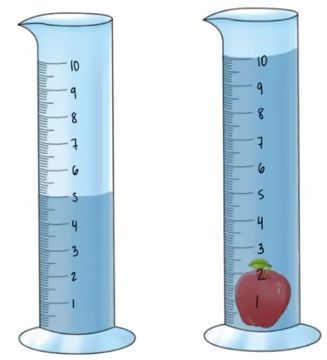
What is the density of an object with a mass of 30 grams and the volume below:
Density= Mass / Volume
Density= 30g/5cm3
Density= 6g/cm3
At which point does the rollercoaster car have the most potential energy? Kinetic energy?
Potential energy: B
Kinetic energy: A
A student places a drop of food coloring into cold water and hot water and observes. Why does the food coloring in hot water disperse faster?
Food coloring disperses faster in hot water because heat gives water molecules more kinetic energy, making them move and collide more rapidly and forcefully, which pushes the dye molecules around quicker.
How many protons, neutrons, and electrons are there in a helium atom?
2 protons
2 electrons
2 neutrons
What is the difference between heterogeneous and homogeneous mixtures?
The main difference is that homogeneous mixtures have a uniform composition throughout, meaning they look the same everywhere (like saltwater), while heterogeneous mixtures have a non-uniform composition, with components that are visibly distinct (like a salad).

Marcus has an unknown substance. He conducts a series of experiments to determine some of its properties. Which experiments tested for physical properties? Which tested for chemical properties?
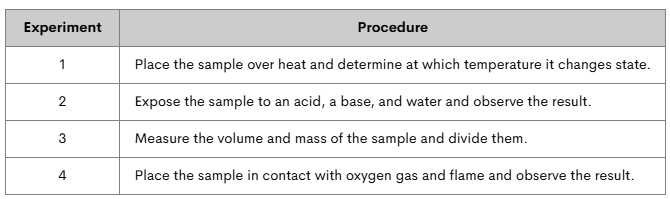
xperiment 1 tests boiling point= physical property.
Experiment 2 tests reactivity= chemical property.
Experiment 3 tests density= physical property.
Experiment 4 tests combustibility= chemical property.
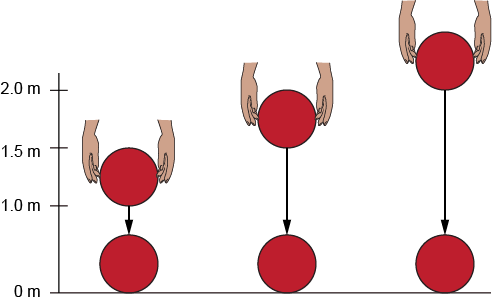
A student is doing a ball-drop experiment. She notices that the higher she drops the ball, the higher it bounces. Why does this happen?
When the ball is dropped from a higher height, it has more potential energy. As it falls, that energy turns into kinetic energy. When the ball hits the ground, the law of conservation of energy says that energy is not lost, it changes form. So the more energy the ball starts with, the more energy it has to bounce back up, which makes it bounce higher.
Observe the cubes in three different scenarios below. Label the heat transfers.
1- Conduction- the cubes are in direct contact.
2- Radiation- the cubes have empty space between them.
3- Convection- the heat of one cube will reach the other through the movement of oxygen gas.
Why are elements placed in the same group on the periodic table?
Elements are grouped on the periodic table because they share similar chemical properties.
If a mystery substance is boiled and as the liquid boils away some particles remain, is this a pure substance or mixture? Why?

It's a mixture, not a pure substance, because pure substances (elements or compounds) boil at a constant, specific temperature and evaporate completely, leaving no residue. The leftover particles indicate impurities or other components with higher boiling points, meaning the substance wasn't uniform and separated during heating, which is the defining characteristic of a mixture.
Define and provide examples of physical and chemical changes.
Physical changes alter a substance's form (shape, size, state) without changing its chemical identity, like melting ice; chemical changes (chemical reactions) create new substances with different properties, such as burning wood to ash, involving bond breaking and new bond formation, and are often hard to reverse.

A hand crank radio is a durable and battery-free radio that uses a hand crank to generate power for listening to AM/FM/weather stations. What energy transformations take place to crank up the radio?

Cranking a hand-crank radio transforms your muscle's chemical energy (from food) into mechanical energy, which spins a coil in a magnetic field (a generator), converting it into electrical energy; this electricity then powers the radio's circuits, which finally transform electrical energy into the sound energy you hear from the speaker.
What will happen to the temperatures of the cubes below? How will heat move?
Thermal energy flows from warmer objects to cooler objects. The warmer cube will cool down as the colder cube will warm up until they are equal.