Shows an exothermic reaction...

B
The mole relationship between N2 and H2.
N2 + 3H2 --> 2NH3
1:3
The ion charge for gallium (atomic number 31).
3+
The strongest of all intermolecular forces.
Hydrogen bonding
In a solution such as Mug Root Beer, sugar is the...
solute
Adding more HCl to the following chemical equation at equilibrium, will shift the reaction towards the...
HCl + Ca(OH)2 ⇋ CaCl2 + H2O + heat
products (right)
How many moles of Al2O3 are required to produce 12 moles of oxygen (O2)?
2Al2O3 --> 4Al + 3O2
8 moles of Al2O3
The formula for calcium nitride.
Ca3N2
A polar molecule must have _____ bonds and be _____ in shape.
polar, lopsided (asymetrical)
In a rigid container, the temperature of a gas is decreased. What happens to the pressure?
decreases
A catalyst _______ the rate of reaction by ________ the activation energy.
increases, decreasing
This is 2 types of reactions...
Zn + O2 --> ZnO
Synthesis (or combination, or composition)
and
Metal Oxidation
The name for P2O3
diphosphorus trioxide
Benzene does not dissolve in water, therefore benzene is ___________ .
nonpolar
25.0 mL of HCl is titrated with 32.6 mL of 0.50 M NaOH. What is the molarity of the HCl?
0.65 M HCl
decreasing the temperature to the following reaction that is at equilibrium will cause it to shift towards the...
CuSO4*H2O + heat ⇋ CuSO4 + H2O
reactants (left)
C3H6 + O2 --> CO2 + H2O
2, 9, 6, 6
2C3H6 + 9O2 --> 6CO2 + 6H2O
What is the shape of the following molecule?
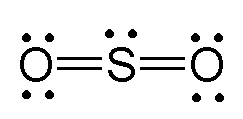
Bent
The dominate intermolecular force in a container full of sulfur difluoride is...

dipole-dipole
745 K
The type of reaction where the amount of energy needed break the reactant bonds is more than the energy released when the products are formed.
Endothermic reaction
How many grams of N2 are required to react with 15 moles of H2?
N2 + 3H2 --> 2NH3
140 grams of N2
What is the shape around the top right carbon in the caffeine molecule?
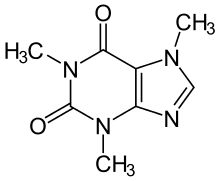
tetrahedral
In a container of CF4 gas, the dominate intermolecular force is...
dispersion
How many moles of oxygen are required to completely react with 56.7 L of hydrogen gas? The reaction proceeds at STP.
2H2 + O2 --> 2H2O
1.27 moles of O2