_________ is the study of matter and how it changes
Chemistry
Weight is a flawed measure of mass because it depends on ____________________.
Gravity
Below is a paragraph describing how to make bread. Name the Chemical Change and the physical change:
After the dry and wet ingredients are mixed, yeast is added to the dough which reacts with the sugars in the mixture to produce bubbles of carbon dioxide. Heat is added to the mixture using an oven. When the bread is fully baked, it is sliced into twenty-two evenly sized pieces.
Physical Change - mixing the ingredients, slicing the bread
Chemical Change - yeast reacting to the sugars, adding heat as a catalyst
The formula for Work
Work = Force x Distance
What word means “in a regular, repeated pattern”?
Periodic
Elements cannot be broken down into smaller substances.
True or False
True
Which would weigh more: a pound of feathers or a pound of sand? __________________
They weigh the same.
If you cut an apple into slices and leave them in the open air, they will slowly turn brown. What kind of chemical change is this? _________________
Oxidation
The formula for Power
Power = Work/Time
Atoms are arranged on the periodic table by increasing ____________________ .
atomic number
Name the two elements that make up this molecule:
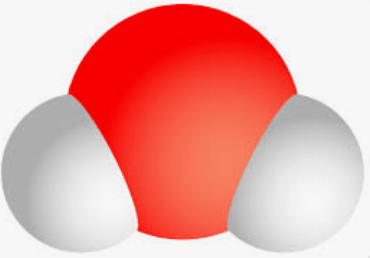
Hydrogen and Oxygen
What formula do you use to determine the volume of an object of regular shape?
Length x Width x Height = Volume
The law that says, "No matter is being created or destroyed, only transformed." This an example of the Law of ______________________ of __________.
The Law of Conservation of Mass
The energy of motion is called ________________________ energy.
Kinetic
Use the periodic table banner in the front of the room, which two elements have the same number of valence electrons?
Silicon and Phosphorus
Neon and Sulfur
Chlorine and Iodine
Sodium and Calcium
Chlorine and Iodine
The little number below an element symbol that indicates how many of that element are present in each molecule is called a _________________________.
Subscript
Looking at this chart, put the items in order from greatest to least density. (If mixed together, in what order would these substances stack from bottom to top.)
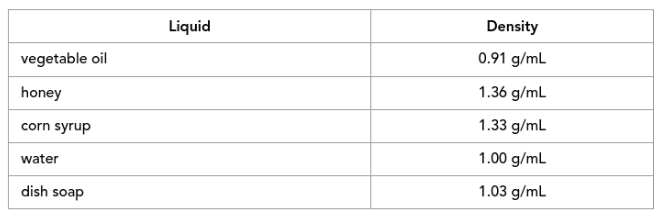
vegetable oil - Top
water
dish soap
corn syrup
honey - Bottom
Give an example of a material that is likely to be damaged by an acid
Which factor, if increased, will affect kinetic energy the most: Speed or Mass
Speed
How many valence electrons does Aluminum have?
How many valence electrons do the Noble gasses have?
3
8
Which atomic particle has a positive charge?
Which atomic particle has a negative charge?
Which atomic particle has no charge at all
Proton
Electron
Neutron
The formula for Density is _________________.
Density = Mass/Volume
Give an example of a material that is likely not to be damaged by an acid
Any nonmetal (glass or plastic)
The formula for Kinetic Energy is KE = ½ x Mass x Speed².
What is the Kinetic Energy of a 40 kg boy running at a speed of 3 m/s? ________________
60 Newtons
How many valence electrons does it take for an atom to be stable?
2 for the first layer (helium and hydrogen)
8 for every other layer (for every other element)
point out the different parts of an atom in diagram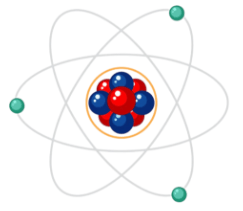
Protons - in the nucleus
Neutrons - in the nucleus
Electrons - orbiting outside
A measure of how much solute can dissolve in a given solution is called a ____________.
solubility
Bases turn litmus paper _________. (red / blue)
While acids turn litmus paper ____________. (red / blue)
blue
red
Where on the roller coaster track to the car have the most stored potential energy? Where does it have the most kinetic energy?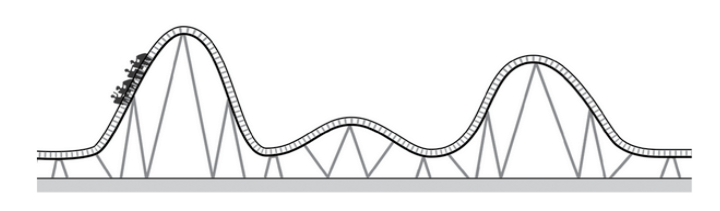
At the very top of the highest hill
At the very bottom of the highest hill
Ionic Compounds have a _____________ melting point. (high / low)
While covalent compounds have a _____________ melting point. (high / low)
High
Low