Which of the following quantitative measures of inspiratory and expiratory flow is especially useful in diagnosing chronic obstructive pulmonary disease (COPD)?
A. Forced expiratory volume in one second (FEV1)
B. Forced vital capacity (FVC)
C. Functional residual capacity (FRC)
D. Peak expiratory flow (PEF)
Answer: A: Forced expiratory volume in one second (FEV1). This, along with FVC (choice B), helps distinguish obstructive from restrictive lung disorders. Choice C: FRC does not measure airflow and is therefore incorrect. Choice D: PEF is used primarily to monitor asthma patients.
Analyze the following ABG:
pH 7.21 / pCO2 51mmHg / pO2 90 / HCO3 21mEq/L
What is the primary problem?
A. Respiratory acidosis
B. Metabolic acidosis
C. Respiratory alkalosis
D. Metabolic alkalosis
E. Combined acidosis
F. Combined alkalosis
Answer: E
This is a combined acidosis. The pH is < 7.35 which indicates an acidosis. The pCO2 is > 40 which indicates a respiratory component to the acidosis. The HCO3 is < 24 which indicates a metabolic component to the acidosis.
A combined acidosis is by definition uncompensated.
A 65-year-old woman is hospitalized for acute pancreatitis due to alcohol use. During hospitalization, she receives lactated Ringer solution overnight and is taking nothing by mouth.
On physical examination the morning after admission, the patient reports improved pain. Vital signs are normal. She has mild epigastric pain without guarding. Bowel sounds are present.
After fluid resuscitation, hematocrit decreases from 45% at admission to 38% 12 hours later.
Which of the following is the most appropriate management?
A. Initiate enteral tube feeding
B. Initiate oral feeding
C. Maintain nothing-by-mouth status
D. Transfuse 2 units of packed red blood cells
The most appropriate management is initiation of oral feeding (Option B). The patient has acute pancreatitis. The diagnosis of acute pancreatitis requires two of the following three criteria: (1) acute-onset abdominal pain characteristic of pancreatitis, (2) serum lipase or amylase levels elevated at least three times the upper limit of normal, and (3) characteristic imaging findings. She received intravenous resuscitation overnight, which typically consists of 250 to 500 mL/h of normal saline or lactated Ringer solution, with fluid boluses as needed for severe volume depletion. Treatment with intravenous fluids should be aimed at avoiding hypovolemia and maintaining adequate organ perfusion. Goal-directed therapy allows for titration of fluids to particular biochemical and clinical targets, including blood pressure, pulse rate, urine output, blood urea nitrogen, and hematocrit. This patient initially had risk factors for severe disease, including a hematocrit greater than 44%, which has improved with volume resuscitation. Overall, her pain is improving and oral feeding should therefore be considered. Patients with acute pancreatitis should resume feeding when pain improves and any nausea or vomiting subsides. Oral feeding is preferred, and guidelines recommend starting oral feeding within 24 hours.
If patients with acute pancreatitis do not tolerate oral feeding, enteral tube feeding (Option A) should be considered within 72 hours. Historically, this approach included placement of a nasojejunal tube to avoid stimulation of the pancreas. More recent data suggest that nasogastric tube placement and feedings are easier to administer than nasojejunal feeding and are as efficacious. In this patient, however, oral feeding should be attempted before enteral feeding is considered.
In the past, management of acute pancreatitis included keeping patients on nothing-by-mouth status (Option C) to prevent stimulation of the pancreas. However, more recent evidence has demonstrated a benefit to early feeding; this approach may promote a healthy mucosal barrier and avoid translocation of gut bacteria, which can result in local and systemic infection.
Transfusion of packed red blood cells (Option D) is not required at this time. Although complications of pancreatitis may include gastrointestinal bleeding (e.g., splenic vein thrombus leading to varices or pseudoaneurysm formation followed by rupture of an arterial wall), this patient is hemodynamically stable and does not exhibit signs or symptoms of acute gastrointestinal bleeding. She was intravascularly depleted at the time of hospitalization, and fluid resuscitation led to an expected decrease in hematocrit.
A 65-year-old man is evaluated after being diagnosed with high-risk prostate cancer. He is asymptomatic. Transrectal ultrasound biopsy showed multiple foci of prostate cancer in both lobes with the highest Gleason score of 8.
On physical examination, vital signs and the remainder of the examination are normal.
CT and bone scan are negative for metastatic disease.
The patient has been started on treatment with androgen deprivation therapy plus radiation therapy.
Which of the following is the most appropriate screening test to perform next?
A. Dementia screening
B. Doppler ultrasonography of the lower extremities
C. Dual-energy x-ray absorptiometry scan
D. Exercise stress test
The most appropriate screening test to perform next for this patient is a dual-energy x-ray absorptiometry (DEXA) scan (Option C) to establish baseline bone density and to assess for fracture risk. Patients diagnosed with high-risk nonmetastatic prostate cancer are usually treated with a combination of androgen deprivation therapy (ADT) and radiation therapy, which has been shown to improve survival. ADT results in many short- and long-term adverse effects. Short-term effects include loss of lean body mass, fatigue, gynecomastia, hair loss, decreased libido, erectile dysfunction, and vasomotor symptoms. Long-term risks include a possible increase in cardiovascular disease and cognitive dysfunction, increased risk of venous thromboembolism, and reduction in bone density. Patients with nonmetastatic cancer with one or more risk factors for osteoporotic fracture should be offered DEXA screening. ADT is a risk factor for osteoporosis. Patients who are prescribed a drug that causes bone loss or whose bone mineral density is near the threshold of treatment should be screened every 2 years. For patients with osteoporosis, bisphosphonates or denosumab may be offered to reduce the risk of fracture.
There is increasing but inconsistent evidence that ADT is associated with cognitive impairment and Alzheimer disease. However, there is no recommendation for routine dementia screening (Option A) in patients taking ATD, and the U.S. Preventive Services Task Force has concluded that there is insufficient evidence for or against routine dementia screening in older adults.
Patients treated with ADT may be at risk for cardiovascular disease and are at increased risk for venous thromboembolism. However, there are no recommendations for screening with either exercise electrocardiography (Option D) or Doppler ultrasonography (Option B) in asymptomatic patients.
Anechoic fluid anterior to the descending aorta in the parasternal long-axis (PLAX) view is consistent with which of the following diagnoses?
Pericardial effusion
Which of the following physiologic changes is most likely indicative of an obstructive pulmonary disorder?
A. FEV1 is decreased; FVC is decreased; TLC is decreased
B. FEV1 is decreased; FVC is decreased; TLC is increased
C. FEV1 is decreased; FVC is increased; TLC is increased
D. FEV1 is increased; FVC is decreased; TLC is increased
Answer: B: FEV1 is decreased; FVC is decreased; TLC is increased. These readings, in addition to decreased FEV1/FVC and normal or increased RV, are indicative of an obstructive pulmonary disorder. Choice A is indicative of restrictive pulmonary disorders. Choice C is not indicative of a pulmonary disorder, and choice D is indicative of a mixed pulmonary disorder.
Analyze the following blood gas:
pH 7.49 / pCO2 23 mmHg / HCO3 17 mEq/L
What is the acid-base disorder?
A. Respiratory alkalosis with partial metabolic compensation
B. Respiratory alkalosis with complete metabolic compensation
C. Metabolic alkalosis with partial respiratory compensation
D. Metabolic alkalosis with complete respiratory compensation
Answer: A
This is a respiratory alkalosis indicated by the pH > 7.45 and the pCO2 < 40. The HCO3 is low (< 24) which shows metabolic compensation. However, it is not complete compensation because the pH remains alkalotic (> 7.45).
A 40-year-old man is evaluated in the emergency department for severe epigastric abdominal pain radiating to the back that has worsened in the past 12 hours.
On physical examination, temperature is 39.4 °C (102.9 °F), blood pressure is 100/60 mm Hg, pulse rate is 110/min, and respiration rate is 28/min. Palpation of abdomen elicits epigastric pain without guarding.
Laboratory studies:
Leukocyte count - 18,000/μL (18 × 109/L)
Alanine aminotransferase - 178 U/L
Aspartate aminotransferase - 145 U/L
Total bilirubin - 1.1 mg/dL (18.8 μmol/L)
Lipase - 100 U/L
Blood and urine cultures are pending.
Fluid resuscitation with lactated Ringer solution is initiated.
Which of the following is the most appropriate initial management?
The most appropriate management is contrast-enhanced CT (Option A). This patient probably has acute pancreatitis, the diagnosis of which requires at least two of the following: (1) acute-onset abdominal pain characteristic of pancreatitis (severe, persistent for hours to days, and epigastric in location, often radiating to the back); (2) elevation in pancreatic enzymes (typically lipase) three times the upper limit of normal or higher; and (3) imaging findings characteristic of acute pancreatitis, including peripancreatic fat stranding and inflammation as well as peripancreatic edema. Serum amylase and lipase levels may be elevated in nonpancreatic conditions (e.g., kidney disease, acute appendicitis) and therefore are not specific for acute pancreatitis. This patient has typical symptoms, but his lipase levels do not meet the threshold for diagnosis. Therefore, cross-sectional imaging is recommended to confirm the diagnosis. Cross-sectional imaging is also helpful to assess patients for other conditions that might mimic acute pancreatitis and to evaluate patients with atypical symptoms. Preferred imaging modalities include contrast-enhanced CT and MRI.
Routine use of antibiotics (Option B) is not warranted in acute pancreatitis unless there is evidence of extrapancreatic infection, such as ascending cholangitis, bacteremia, urinary tract infection, or pneumonia. Prophylactic antibiotics do not affect the rates of important outcomes, such as organ failure and hospital length of stay.
Endoscopic ultrasonography (Option C), in which an ultrasound probe is placed in the stomach and small intestine to better visualize the pancreas and biliary system, is not typically used in the initial diagnosis of pancreatitis because of its invasive nature.
Once a diagnosis of pancreatitis is confirmed, patients should undergo an assessment for the cause of acute pancreatitis. Biliary disease and alcohol are the most common causes of acute pancreatitis. Transabdominal ultrasonography is the preferred imaging modality to assess for a biliary cause of acute pancreatitis. Triglyceride levels (Option D) should be measured in patients without a biliary cause of acute pancreatitis; a triglyceride level exceeding 1000 mg/dL (11.3 mmol/L) can be considered the cause of the acute pancreatitis. Because the diagnosis of acute pancreatitis has not been established and the biliary tree has not been evaluated, measurement of triglyceride levels is premature.
A 65-year-old man is evaluated for a single episode of painless gross hematuria that occurred 1 month ago. Medical history is notable only for atrial fibrillation managed with metoprolol and rivaroxaban.
On physical examination, vital signs are normal. Cardiac examination reveals an irregularly irregular rhythm.
Urinalysis is normal.
Ultrasound of the kidneys and bladder is normal.
Which of the following is the most appropriate management?
A. Cystoscopy
B. Repeat urinalysis in 1 week
C. Substitute warfarin for rivaroxaban
D. Urine cytology
E. Reassurance and no additional intervention
This patient requires prompt referral to a urologist for cystoscopy (Option A) in the evaluation of unexplained gross hematuria. Hematuria is defined as the presence of ≥3 erythrocytes/hpf in the urine sediment and may be microscopic (detectable only on urine testing) or macroscopic (grossly visible). A single incidental finding of hematuria is sufficient to warrant further investigation. Evaluation should be pursued even in patients with bleeding diatheses, or in those taking antiplatelet or anticoagulation therapy. If menstruation, viral illness, vigorous exercise, or some other benign cause is suspected, urinalysis should be repeated after the cause is resolved. If infection is confirmed, urinalysis should be repeated after treatment to document resolution of hematuria. Although CT urography (contrast CT with kidney-specific imaging) has the highest sensitivity and specificity for renal malignancy, noncontrast helical CT is more appropriate if a kidney stone is suspected. Ultrasonography is a reasonable first imaging step because of availability, lower cost, and no ionizing radiation. Cystoscopy is indicated when imaging results are negative.
Discontinuing rivaroxaban and choosing warfarin (Option C) as an alternative anticoagulant is not appropriate. Gross hematuria requires a thorough evaluation regardless of the use of anticoagulant therapy. Finally, there is no evidence that warfarin is any safer than rivaroxaban in preventing hematuria.
The American College of Physicians and the American Urological Association recommend against obtaining urine cytology (Option D) in the initial evaluation of hematuria due to poor sensitivity.
Reassurance without additional intervention (Option E) or repeating a urinalysis (Option B) is not appropriate, as a single episode of gross hematuria should trigger an evaluation for the cause.
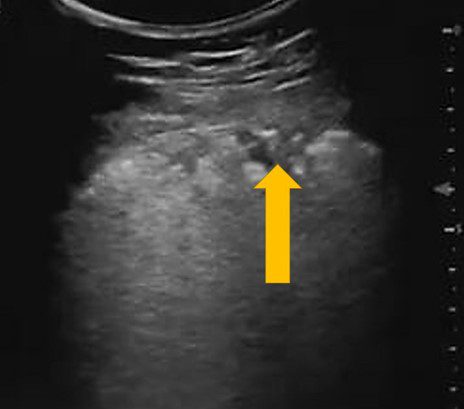
The image above was obtained in Zone 3 of a patient with confirmed a COVID-19 polymerase chain reaction test (PCR).
What lesion is the yellow arrow pointing to?
A. Pleural effusion
B. Pulmonary infarct
C. Subpleural consolidation
C
The arrow is pointing to a pleura based hypoechoic lesion. The findings are consistent with subpleural consolidation. These lesions have been observed in COVID-19 patients but are not specific to COVID-19. No anechoic collection is seen.
Your patient, a 60 year old male smoker, presents with a six month history of progressive shortness of breath and reduced exercise tolerance. You perform bedside spirometry, which yields the following results:
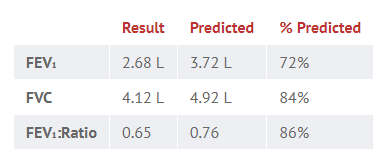
What is the most likely diagnosis?
A. Asthma
B. COPD
C. Interstitial lung disease
D. Pulmonary embolus
Answer: B
The FEV₁:FVC ratio is less than 0.7, which is diagnostic of obstructive disease. Given the smoking history and age of onset, the most likely cause is chronic obstructive pulmonary disease (COPD).
A 26-year-old man has been short of breath for 12h and is feeling generally unwell. An arterial blood gas is taken (on room air): pH 7.51 / PCO2 23 / PO2 67 / HCO3– 23.1mmol/L.
Which is the single most likely diagnosis?
A. Severe vomiting
B. Methanol overdose
C. Pulmonary embolus
D. Diabetic ketoacidosis
E. Panic attack
Answer: C
This man has become acutely breathless from a PE. He is hypoxic and, as a reflex to this, is hyperventilating (as evidenced by the low PaCO2). As a result, he has developed an alkalosis. Note: PEs may present differently depending on extent of hypoperfusion and RR. Diabetic ketoacidosis & Methanol overdose: These are both causes of a metabolic acidosis (with a raised anion gap). Panic attack: This does cause acute alkalosis via hyperventilation (and therefore low PaCO2 and a high pH), but tends to happen in the absence of hypoxia rather than as a response to it (as in pulmonary embolism). Severe vomiting causes a metabolic alkalosis (i.e. a high pH with a high HCO3–).
A 74-year-old woman is evaluated in the emergency department for yellow skin, dark urine, and light stools of 10 days' duration.
On physical examination, vital signs are normal. Jaundice and icteric sclerae are noted.
Laboratory studies:
Alkaline phosphatase - 175 U/L
Alanine aminotransferase - 57 U/L
Aspartate aminotransferase - 49 U/L
Total bilirubin - 6.0 mg/dL (102.6 μmol/L)
Direct bilirubin - 4.6 mg/dL (78.7 μmol/L)
CT reveals retroperitoneal fibrosis and a “sausage-shaped” pancreas with a narrowed pancreatic duct. Endoscopic ultrasound with fine-needle aspiration is negative for malignancy but shows more than 10 IgG4-positive cells/hpf.
Which of the following is the most appropriate management?
A. Azathioprine
B. Endoscopic retrograde cholangiopancreatography with biliary stent placement
C. Prednisone
D. Total pancreatectomy.
The most appropriate management is prednisone (Option C). The patient has painless jaundice with imaging features suggestive of type 1 autoimmune pancreatitis (AIP). CT findings that strongly suggest type 1 AIP include a diffusely enlarged pancreas with indistinct borders and delayed contrast enhancement. In comparison, imaging characteristics of pancreatic cancer include a low-density mass and dilatation and/or compression of the pancreatic duct associated with distal pancreatic atrophy. Endoscopic ultrasound with fine-needle aspiration, if pursued, shows greater than 10 IgG4-positive cells/hpf; this finding is consistent with a diagnosis of type 1 AIP, a manifestation of IgG4 disease. A significant elevation of serum IgG4 level is also helpful in diagnosing type 1 AIP. Most IgG4-related conditions are characterized by plasma cell infiltration of the tissues and subsequent clinical manifestations. AIP is a frequent manifestation of IgG4-related disease, but nearly any organ may be involved, including lymph nodes, salivary glands, and the biliary system. Retroperitoneal fibrosis may also be related to IgG4 disease. Initial treatment includes high-dose prednisone tapered over 2 to 3 months. Symptoms typically resolve within 2 to 4 weeks of therapy. Approximately 90% of patients achieve remission with oral glucocorticoids. Failure of clinical symptoms to respond to glucocorticoids suggests an incorrect diagnosis, and other causes should be investigated. Up to 60% of patients may relapse. Readministration of glucocorticoids or immunomodulators, such as 6-mercaptopurine, azathioprine, mycophenolate, or rituximab, may be used to treat recurrent AIP. Diagnostic criteria for AIP are known by the acronym HISORt (diagnostic Histology, suggestive Imaging, Serology with elevated serum IgG4, Other organ involvement, or Response to therapy with glucocorticoids). Type 2 AIP, or idiopathic duct-centric pancreatitis, does not demonstrate IgG4-positive cells but is instead characterized by granulocytic lesions. Type 2 AIP may be associated with inflammatory bowel disease.
The immunosuppressant azathioprine (Option A) may be used as a glucocorticoid-sparing agent to treat relapsing IgG4-related disease. Because of its slow onset, azathioprine is not used for induction therapy but is useful in the maintenance phase of therapy for recurrent disease.
Endoscopic retrograde cholangiopancreatography with biliary stent placement (Option B) is not required in AIP because jaundice should respond to glucocorticoids.
Although pancreatic adenocarcinoma and autoimmune pancreatitis may be mistaken for each other, in this patient the diagnosis appears certain on the basis of imaging characteristics and needle biopsy results. Total pancreatectomy (Option D) for pancreatic carcinoma is not indicated.
A 72-year-old man was diagnosed 9 years ago with prostate cancer. After radiation treatment, his prostate-specific antigen (PSA) level dropped to a nadir of 1.5 ng/mL (1.5 µg/L); it had remained stable until it rose to 2 ng/mL (2 µg/L) 1 year ago and is currently 3.7 ng/mL (3.7 µg/L). He has no symptoms. Medical history is unremarkable, and he takes no medications.
Vital signs and all other physical examination findings are normal. Results of all other laboratory studies are within normal limits.
Biopsy of the prostate is negative. CT of the chest, abdomen, and pelvis and bone scan are negative for metastatic disease.
Which of the following is the most appropriate management?
A. Androgen deprivation therapy
B. Chemotherapy
C. Cryotherapy
D. Monitor PSA level without treatment
Correct Answer: D
This patient has a prostate-specific antigen (PSA)–only recurrence of prostate cancer, and the most appropriate management is to monitor PSA level without treatment (Option D). Men treated with radiation therapy rarely achieve an undetectable PSA, as is commonly seen after surgery. Rather, the PSA will fall with treatment and reach a nadir. PSA-only recurrence is defined as an increase in the PSA level by at least 2 ng/mL (2 µg/L) above the postradiation therapy nadir. This patient had a nadir PSA of approximately 1.5 ng/mL (1.5 µg/L) and now has a PSA of 3.7 ng/mL (3.7 µg/L) 9 years after treatment. Imaging studies did not reveal any evidence of metastatic disease. Therefore, he has a PSA-only recurrence. Treatment for patients with PSA-only recurrence is informed by the degree of PSA elevation and the rate of rise in the PSA level. For men with a rapid doubling time, defined as 10 months or less, treatment is generally recommended. Standard treatment in this setting is an androgen receptor blocker (such as enzalutamide). Men with a slow PSA doubling time (for example, more than 10 months) do not require immediate treatment, however, as it can take years for them to develop clinical metastatic disease. They can be monitored with serial PSA assessment, and treatment can be deferred.
Androgen deprivation therapy (Option A) is not indicated in this case, given the slow PSA doubling time and the absence of biopsy-positive recurrent or metastatic disease.
Chemotherapy (Option B) with docetaxel is reserved for treatment of men with symptomatic metastatic disease. This patient does not have metastatic disease and has no symptoms. There is no reason to subject him to the toxicity of chemotherapy when he does not require treatment.
Cryotherapy (Option C) is not indicated in this case. This patient underwent transrectal ultrasound-guided biopsy to assess for focal lesions in the prostate amenable to local therapy. Given that biopsy result was negative, there is no role for cryotherapy or other local treatment to the prostate.
A 45-year-old Hispanic female presented to the Family Medicine clinic with a history of fever, chest pain and dyspnea for 7 days. The physician performed a physical exam and then proceeded to perform a POCUS lung examination. The above view was obtained.
Which of the sonographic findings is seen in the image below?
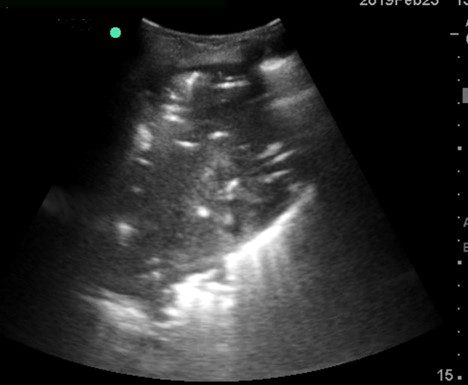
A. Air bronchograms
B. Jellyfish sign
C. Barcode sign
A.
When the alveoli are filled with fluid/secretions, the affected lung area can be seen on ultrasound as the fluid/secretions allow the ultrasound beam to travel through it. If the bronchial tree still has air in it then we see echogenic areas within the lung parenchyma. These are known as sonographic air bronchograms. These could be tiny punctate lesions or could be longer and linear. If the air bronchogram is not moving during respiration it is known as a static air bronchogram. This indicates the presence of trapped air and is consistent with resorptive atelectasis. However, if the echogenic air bronchogram is moving with respiration, then it indicates the presence of a non-retractile consolidation and is known as a dynamic air bronchogram and is most probably due to a pneumonia (94% specificity).
A 24-year-old woman is evaluated for intermittent cough, wheezing, and chest tightness of 1 month's duration. She reports worsening symptoms with exercise and with cat exposure.
On physical examination, vital signs are normal. She has end-expiratory wheezing. Cardiac examination is normal.
On spirometry, FEV1 is 75% of predicted and improves significantly following inhaled albuterol.
Which of the following tests will help predict this patient's responsiveness to inhaled glucocorticoids?
A. Bronchial challenge testing
B. Diffusing capacity for carbon monoxide
C. Fractional exhaled nitric oxide
D. Pulse oximetry
C. Fractional exhaled nitric oxide
Fractional exhaled nitric oxide (FeNO) (Option C) will help predict this patient's responsiveness to inhaled glucocorticoids. Although FeNO should not be used as a diagnostic tool for asthma, it can be used to support this diagnosis in situations in which additional objective evidence is needed. FeNO may be useful if there is uncertainty in choosing, monitoring, or adjusting anti-inflammatory therapies based on history, clinical findings, and spirometry as part of an ongoing asthma monitoring and management strategy. In adults with respiratory symptoms, FeNO levels above 50 ppb correlate with eosinophilic airway inflammation and predict response to inhaled glucocorticoids. FeNO levels below 25 ppb indicate that eosinophilic airway inflammation and glucocorticoid responsiveness are less likely; values between 25 and 50 ppb should be interpreted cautiously. In patients with asthma that is being treated with inhaled glucocorticoids, serial measurement of FeNO may help to monitor patient response to glucocorticoid therapy.
Bronchial challenge testing (Option A) is used to identify bronchial hyperresponsiveness, a diagnostic feature of asthma. This is particularly helpful in patients whose symptoms are suggestive of asthma but for whom other pulmonary function test results are normal. Patients inhale increasing doses of a substance known to induce bronchospasm, such as methacholine or histamine, in a stepwise fashion. This is followed by repeated measurements of FEV1; if FEV1 falls by 20% or more from the baseline value, the test is considered positive.
Diffusing capacity for carbon monoxide (DLCO) measurement (Option B) estimates the amount of gas transfer through the alveolar/capillary unit and is proportional to the surface area of a functional lung. DLCO is measured by inhalation of a gas mixture containing carbon monoxide and helium; the resulting value is corrected for hemoglobin level. DLCO is reduced in conditions in which functioning alveolar capillary units are destroyed, infiltrated, removed, or their function is compromised. Conditions that increase pulmonary capillary blood volume, such as pulmonary alveolar hemorrhage, left-to-right shunt, or asthma, can cause an elevation in DLCO. DLCO measurements do not predict responsiveness to glucocorticoids.
Pulse oximetry (Option D) provides a readily available noninvasive measurement of oxygen-bound hemoglobin in the circulation. A normal hemoglobin saturation measured by pulse oximetry is 95% to 100%, and values below 90% indicate hypoxemia. Pulse oximetry cannot predict responsiveness to glucocorticoids.
A 66-year-old woman is evaluated in the ICU for management of acute hypoxic respiratory failure due to viral pneumonia. She has no other medical problems and takes no medications.
On physical examination, the patient is calm, alert, and interactive. Temperature is 37.6 °C (99.7 °F), blood pressure is 140/90 mm Hg, pulse rate is 100/min, and respiration rate is 32/min. Oxygen saturation is 89% breathing oxygen, 3 L/min by nasal cannula. Lung examination reveals bilateral rhonchi and tachypnea. Cardiac examination is normal.
On arterial blood gas analysis with the patient breathing ambient air, pH is 7.40, PCO2 is 37 mm Hg (4.9 kPa), and PO2 is 58 mm Hg (7.7 kPa).
Chest radiograph shows bilateral opacities.
Therapy is initiated.
Which of the following is the most appropriate additional management of the patient's hypoxemia?
A. Intubation and mechanical ventilation
B. No change in current management
C. Noninvasive positive pressure ventilation
D. Oxygen by high-flow humidified nasal cannula
The most appropriate next step in managing this patient's hypoxemia is oxygen by high-flow nasal cannula (HFNC) (Option D). HFNC mixes and humidifies high-flow air and oxygen (30 L/min or more) to deliver a consistent FIO2 (0.21-1.00) through a nasal cannula. The high flow creates positive airway pressure; the amount depends on the flow rate and cannot be measured or monitored consistently. A systematic review of oxygen delivered by HFNC versus standard oxygen therapy for hypoxemic respiratory failure showed that HFNC may decrease rates of endotracheal intubation and has no demonstrated effect on mortality.
Intubation and mechanical ventilation (Option A) are acceptable interventions to manage hypoxemic respiratory failure. However, alternative options such as HFNC and noninvasive ventilation may be preferable because they are less invasive and less prone to complications of therapy if monitored correctly. This patient can maintain gas exchange, has acceptable breathing effort (she is calm and is not using accessory muscles to breathe), and can communicate and cooperate. Thus, HFNC should be pursued first instead of intubation and mechanical ventilation.
Management of hypoxemic respiratory failure centers on administration of supplemental oxygen with the lowest possible FIO2 necessary to meet oxygenation goals. A therapeutic target for peripheral arterial saturation (SpO2) is between 90% and 96%, if feasible. This patient requires additional management to reach that therapeutic target; therefore, no change in hypoxemia management (Option B) is inappropriate.
Evidence favors the use of noninvasive positive pressure ventilation (NPPV) (Option C) in patients with COPD exacerbations, cardiogenic pulmonary edema, neuromuscular disease, and obesity hypoventilation syndrome and in patients who have been extubated, which places them at high risk of morbidity.
A 55-year-old man is evaluated at a follow-up visit. Two months ago he was admitted to the ICU for severe acute pancreatitis from alcohol use. CT during hospitalization revealed an edematous pancreas with fat stranding and peripancreatic fluid collections. He was discharged after 12 days. Two weeks before follow-up he had abdominal pain, and contrast-enhanced pancreas-protocol CT was performed. The scan demonstrated several hypodense lesions throughout the pancreas, including a 12-cm hypodense structure with a well-defined wall and no solid debris. The pancreatic duct was normal and no mass was identified. Today the patient reports feeling well; he has abstained from alcohol since hospitalization.
On physical examination today, vital signs and other findings are normal.
Which of the following is the most appropriate management?
A. Endoscopic cystogastrostomy and necrosectomy
B. Endoscopic retrograde pancreatography
C. Endoscopic ultrasonography with fine-needle aspiration
D. Surgical drainage
E. Observation
The most appropriate management is observation (Option E). This patient has recovered from alcohol-induced pancreatitis and now has a pancreatic pseudocyst. Pancreatic pseudocysts are one of the four types of fluid collections seen in acute pancreatitis; the others are acute peripancreatic fluid collections, acute necrotic collections, and walled-off necrosis. Pancreatic pseudocysts are defined as peripancreatic fluid collections that persist beyond 4 weeks. They develop a well-defined wall, but this wall lacks the epithelial layer required of a true cyst. Pseudocysts do not contain solid material or debris and do not require drainage unless the patient is symptomatic or the pseudocysts become infected. This patient feels well and thus does not require drainage. Ongoing expectant management and observation are appropriate.
Acute necrotic collections are areas of pancreatic necrosis that develop within the first 4 weeks of acute pancreatitis. Necrosis may occur within the pancreatic parenchyma or in the peripancreatic tissues. Walled-off necrosis occurs when necrotic areas liquefy and become encapsulated with a well-defined wall surrounding the necrotic area. Because of the semi-solid nature of the debris in walled-off necrosis, these lesions are not typically amenable to simple endoscopic needle drainage and may require endoscopic drainage, such as endoscopic cystogastrostomy and necrosectomy (Option A), or surgical drainage. Because this patient has a pseudocyst, he does not require these procedures.
Endoscopic retrograde pancreatography (Option B) is not recommended for pancreatic fluid collections. Instrumentation of the pancreatic duct carries a risk for pancreatitis. Although this procedure may provide information, such as connection of the fluid collection to the pancreatic duct, this information will not change management. Therefore, the procedure is not appropriate for this patient.
If a patient is symptomatic or there is concern for infection, endoscopic ultrasonography with fine-needle aspiration (Option C) can be considered. For symptomatic pancreatic pseudocysts, endoscopic drainage is preferred over surgical drainage (Option D) given its lesser morbidity. This patient is asymptomatic and thus does not need to undergo ultrasonography and fine-needle aspiration.
A 60-year-old woman is evaluated for right-sided mid back pain of 2 months' duration. Medical history is significant for hypertension treated with hydrochlorothiazide. She takes no other medications.
On physical examination, vital signs are within normal limits. Oxygen saturation is 99% with the patient breathing ambient air. The remainder of the examination is normal.
Laboratory results:
Hemoglobin - 20 g/dL (200 g/L)
Leukocyte count - 7200/µL (7.2 × 109/L)
Platelet count - 295,000/µL (295 × 109/L)
Urinalysis: 3+ blood; no protein; 30 erythrocytes/hpf; no casts or dysmorphic erythrocytes
Subsequent measurement of erythropoietin level was 180 mU/mL (180 U/L) (elevated)
Which of the following is the most likely diagnosis?
A. Lung cancer
B. Myelodysplastic syndrome
C. Polycythemia vera
D. Relative erythrocytosis
E. Renal cell carcinoma
Correct Answer: E
The most likely diagnosis is renal cell carcinoma (Option E). This patient has secondary erythrocytosis defined by the presence of erythrocytosis and an elevated erythropoietin level. Secondary erythrocytosis may be due to a variety of conditions including systemic hypoxia, high altitude, chronic carbon monoxide exposure, and renal artery stenosis. It may also be caused by autonomous production of erythropoietin independent of hypoxemia, such as a paraneoplastic syndrome. Many different paraneoplastic syndromes can be seen in patients with renal cell carcinoma, including anemia, hepatic dysfunction in the absence of liver metastases (known as Stauffer syndrome), fever, hypercalcemia, AA amyloidosis, thrombocytosis, polymyalgia rheumatica, and erythrocytosis. Many of these conditions can improve with resection of the primary tumor, metastatic sites, or both. An abdominal ultrasound will likely reveal a renal mass that may have sonographic features pathognomonic for renal cell carcinoma. If the ultrasound is equivocal, CT should be performed, typically revealing findings specific enough for renal cell cancer to avoid the need for a confirmatory biopsy.
Lung cancer (Option A) may be associated with a variety of paraneoplastic syndromes, including hypercalcemia and the syndrome of inappropriate antidiuretic hormone secretion, but it has not been associated with ectopic erythropoietin production and would not be expected to cause hematuria.
Myelodysplastic syndrome (Option B) is a stem cell disorder that results in ineffective hematopoiesis and various cytopenias, the most common of which is anemia, often macrocytic. Myelodysplastic syndrome is not a cause of erythrocytosis.
Polycythemia vera (Option C) is a myeloproliferative disorder that is caused by a mutation in the JAK-2 gene. This disorder results in unregulated blood cell production. Although erythrocytosis may be most striking, leukocytosis and thrombocytosis are often seen as well. Physical findings include hepatosplenomegaly. The dysregulated erythrocyte production and erythrocytosis cause a marked reduction in erythropoietin secretion. Hematuria would not be expected.
Relative erythrocytosis (Option D) occurs in patients who have intravascular volume contraction, often in the setting of diuretic use. These patients are characteristically obese, have hypertension, and smoke cigarettes. Relative erythrocytosis would not cause the dramatic hemoglobin elevation seen in this patient and would not explain the hematuria.
A 55-year-old Hispanic female presented to the Family Medicine clinic with a chief complaint of right upper quadrant (RUQ) pain for the past 3 months. She also complained that she is not able to tolerate fatty or fried food. There is no history of trauma or surgery. A POCUS right upper quadrant (RUQ) exam was performed to rule out the possibility of gallbladder stones or some other pathology in the right upper quadrant region. The image above is one of the images obtained during the point-of-care ultrasound exam.
What is the most likely diagnosis?
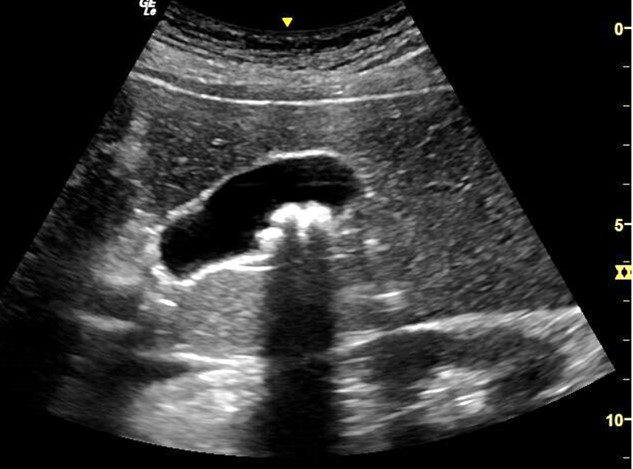
A. Acute cholecystitis
B. Chronic cholecystitis
C. Multiple gallstones
The image shows a well distended gallbladder with multiple hyperechoic stones casting a posterior acoustic shadow in the region of the body of the gallbladder. No stone was seen impacted in the neck of the gallbladder. Gallbladder wall is not thickened. No pericholecystic fluid seen. Thus, the possibility of cholecystitis is ruled out.
A 70 year old male presents with a complaint of dyspnea after eating.

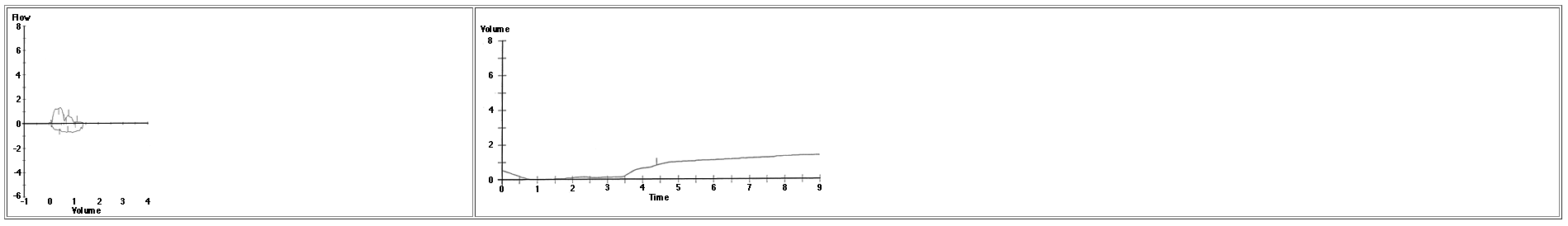
What is the most likely diagnosis?
A. Severe obstructive ventilatory defect, cannot exclude a concomitant restrictive defect
B. Normal, but reduced midrange flows suggest possible small airways disease
C. Flow volume loop suggests a fixed upper airway obstruction
D. Uninterpretable, does not meet acceptability criteria
Answer: D
The volume-time plot reveals a delayed start to the test, an
irregular upstroke on expiration, and a poorly sustained plateau that
all indicate a poor effort . This spirogram is uninterpretable as it
fails to meet acceptability criteria.
And REMEMBER: You cannot make a diagnosis from PFTs alone!
A patient is admitted in septic shock. Her pH on blood gas is 7.28 and her HCO3 is 10. If she is appropriately compensating, what do you expect her pCO2 to be?
Answer: 21-25
We can use Winter's formula to calculate what the pCO2 should be for respiratory compensation in metabolic acidosis.
Winter's formula: Expected pCO2 = (1.5 x serum HCO3) + 8 +/- 2
For this patient: Expected pCO2 = (1.5 x 10) + 8 +/- 2
A 65-year-old woman is evaluated at a follow-up visit. She presented to the emergency department 2 weeks ago with a 2-day history of left-lower-quadrant abdominal pain. CT revealed diverticulitis and a 15-mm cystic structure in the body of the pancreas. Her acute diverticulitis has resolved, and she now feels well. Colonoscopy is scheduled in 6 weeks. She has no other medical conditions and takes no medications. The radiology report indicates that the pancreatic cyst has all the imaging features of a serous cystadenoma.
Vital signs and other physical examination findings are normal.
Which of the following is the most appropriate management?
A. Endoscopic ultrasonography and fine-needle aspiration
B. MRI
C. Surgical resection
D. No further evaluation or intervention
The most appropriate management for the patient's serous cystadenoma is no further evaluation or intervention (Option D). Characteristic findings of serous cystadenomas include multicystic, lobulated structures (sometimes described as a “bunch of grapes”), which may have a central fibrosis scar or calcification. Pancreatic cysts are being detected more frequently because of increased use of imaging and improved imaging techniques; these cysts may be found in 15% of individuals undergoing abdominal imaging. Cystic neoplasms of the pancreas are subcategorized as mucin-producing and non–mucin-producing cysts. Mucin-producing cysts, including intraductal papillary mucinous neoplasms and mucinous cystic neoplasms, are thought to have malignant potential, but many never become malignant. Non–mucin-producing cysts, such as a serous cystadenoma, have no malignant potential and can often be identified by their characteristic imaging features. These cysts require no further evaluation unless symptomatic.
For patients in whom the diagnosis is unclear, endoscopic ultrasonography and fine-needle aspiration (Option A) can be performed for cytology, measurement of carcinoembryonic antigen level, and DNA analysis.
In the absence of high-risk features (e.g., main pancreatic duct dilation, cysts 3 cm or larger, change in the main duct diameter with distal parenchymal atrophy, and association with a solid mass), patients with mucinous cysts may undergo MRI surveillance (Option B), the frequency depending on many factors, including cyst size.
Surgical resection (Option C) of high-risk cysts is the only treatment option. In addition to the high-risk features just described, cysts in patients presenting with obstructive jaundice are also high risk. Surgical resection is recommended for nearly all mucinous neoplasms and main-duct intraductal papillary mucinous neoplasms if the patient is an appropriate surgical candidate. Mucinous cystic neoplasms occur almost exclusively in women in their fifth to seventh decades of life and are almost always located in the body or tail of the pancreas. These neoplasms have moderate malignant potential. Intraductal papillary mucinous neoplasms (IPMNs) are equally prevalent in men and women, usually appearing in their fifth to seventh decades of life. Branch-duct IPMNs are characterized by cystic structures that may appear throughout the pancreas. Imaging characteristics of main duct IPMNs include main pancreatic duct dilation and parenchymal atrophy. These neoplasms have variable malignant potential.
A 51-year-old man is evaluated following biopsy of the prostate gland. His father died of prostate cancer at the age of 60 years, and his mother was diagnosed with breast cancer at the age of 45 years.
Biopsy of the prostate revealed adenocarcinoma with bilateral gland involvement; his Gleason score was 9. Bone scan confirmed multiple osseous metastatic lesions.
Which of the following is the most appropriate management?
A. Cystoscopy
B. PET/CT
C. Prostate-specific antigen density measurement
D. Referral to a genetic counselor
Correct Answer: D
This patient should be referred to a genetic counselor to discuss genetic testing for BRCA1 and BRCA2 mutations (Option D). He has been diagnosed with metastatic prostate cancer. His biopsy revealed high-risk disease based on a Gleason score of 9. Genetic testing for BRCA gene mutation should be done in all men with high-risk disease, including patients with a Gleason score >7, positive lymph nodes, or metastatic disease. The risk of an underlying mutation in patients with metastatic disease is 11.8%. A family history of breast cancer in a first-degree relative diagnosed before the age of 50 years is also an indication for genetic counseling and BRCA testing. Therefore, based on both personal and family history, this patient is clearly a candidate for BRCA testing.
Cystoscopy (Option A) is used to evaluate patients suspected of having bladder cancer or symptoms, such as hematuria, that might indicate bladder wall involvement by an adjacent neoplasm. Cystoscopy would not be done to evaluate otherwise asymptomatic prostate cancer.
PET/CT (Option B) is not indicated for this patient, as he already has evidence of metastatic disease. PET/CT will not change his management and is not a standard test in this setting.
Prostate-specific antigen (PSA) density (Option C) is the PSA divided by prostate volume. This is calculated to correct the PSA for differences in prostate volume between different patients. After definitive treatment for localized prostate cancer, PSA density is used to help with decision-making regarding active surveillance and treatment. This is especially true for asymptomatic men with PSA-only recurrence, as it can take several years for clinical metastatic disease to develop in that setting. PSA density measurement has no role in the evaluation of men with clinical metastatic disease.
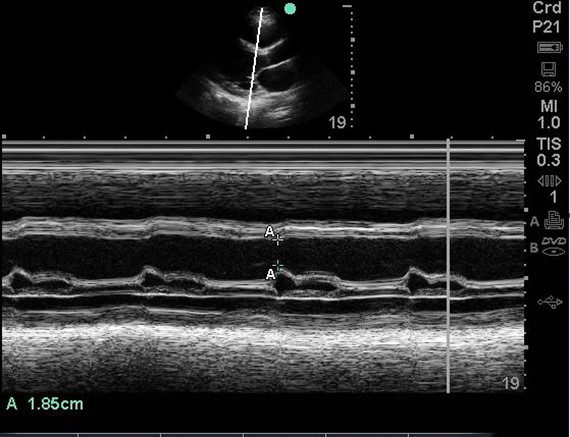
A 75-year-old male with previous medical history of hypertension (HTN), diabetes mellitus (DM) type II and ongoing tobacco use presented with complaint of worsening dyspnea, orthopnea, weight gain and lower extremity edema. The patient also has a family history of ischemic heart disease, cardiovascular accident (CVA).
Temperature - 98.4 degrees Fahrenheit/36.9 C, HR - 68 BPM, BP – 137/84 mm Hg, RR – 20 per minute. EKG shows – Q waves in inferior and lateral leads. No ST-T changes.
Physical exam is remarkable for jugular vein distention (JVD), rales / crackles in lung bases, 1+ pitting edema up to the lower leg (bilateral). A POCUS cardiac exam was performed. An E-Point Septal Separation (EPSS) measurement was obtained.
What is the approximate ejection fracture (EF) range based upon the EPSS measurement?
A. The EF is approximately 50%
B. The EF is less than 30%
C. The EF is more then 60%
B.
A study using MRI data by Jay R Silverstein et al proposed that the mitral valve E point septal separation (EPSS) can be used to quantify LV ejection fraction on a continuous scale. They proposed the following formula to estimate EF on a continuous scale rather than just saying normal or reduced EF.
MRI LVEF = 75.5 – (2.5 x EPSS [millimeters])
Based upon the above equation, the EPSS is 29.25%. It agrees with the chart above as well. Keep in mind that the formula is for calculations using MRI data. But we can get an approximate idea.
Note that this is still an approximate estimation of the EF. True EF can only be extrapolated from volumetric data.