What is the difference between electron affinity and electronegativity?
Electron Affinity: the amount of energy released when an atom gains an electron
Electronegativity: the attraction for electrons WITHIN a chemical bond
Define what anions and cations are
Anions: electrons are added, making the compound negatively charged
Cations: electrons are removed, making the compound positively charged
Write the name for the following compound: IF7
Iodine Heptafluoride
Be happy with two bonds, so no lone pairs on Be
What is the electron and molecular geometry for an element with 6 electron dense areas and 1 lone pair?
Octahedral, Square Pyramidal
What is the Effective Nuclear Charge (Zeff) for the following element:
Iodine (I)
Iodine: 53-46 = 7
Classify the following bonds as either ionic, polar covalent, or nonpolar covalent
Ca-O
Be-Si
B-Br
Ca-O: 3.5-1.0=2.5 ionic
Be-Si: 1.8-1.5: 0.3 nonpolar
B-Br: 2.0-2.8: 0.8 polar covalent
What is the name of these 3 compounds?
NH4NO3
Ag3(PO4)2
PCl4
Ammonium Nitrate
Silver (II) Phosphate
Phosphorus Tetrachloride
Draw the lewis structure for CH3OH
Oxygen connected to the carbon, last hydrogen connected to the oxygen
Draw the correct lewis structure for ClF3 and determine the following:
Both geometries, hybridization, and polarity

Trigonal Bipyramidal, T-Shaped, sp3d, polar
What period 3 element has the following ionization energies:
IE1 = 790
IE2 = 1600
IE3 = 3200
IE4 = 4400
IE5 = 16100
IE6 = 19800
Name at least 2 elements that break the octet rule other than hydrogen
Incomplete: Boron and beryllium...
Expanded: Phosphorus, sulfur, chlorine, bromine, iodine, xenon, arsenic, selenium...
Write the 10 prefixes you are expected to memorize
mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca
Draw the lewis structure for SO3
Double bonds on each sulfur
How many sigma (σ) bonds and pi (π) bonds are there in the benzene molecule?
12 sigma bonds
3 pi bonds
Rank the following elements in terms of increasing atomic radius
Sr, Mg, Ca
Sb, Te, I
Mg < Ca < Sr
I < Te < Sb
Classify the following compounds as either ionic or molecular:
CO2
NiN2
CaF2
B2O6
CO2 molecular
NiN2 ionic
CaF2 ionic
B2O6 molecular
Write the compound for the following name:
Cobalt (III) Sulfate
CO2(SO4)3
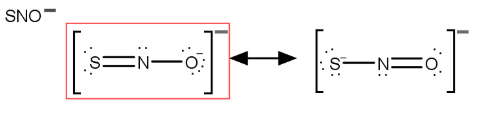
What is the best resonance structure for SNO-?
This is the structure of retinol or vitamin A1. Determine the hybridization of the oxygen atom and the second nearest carbon to the oxygen atom.
Oxygen: sp3
Carbon: sp2
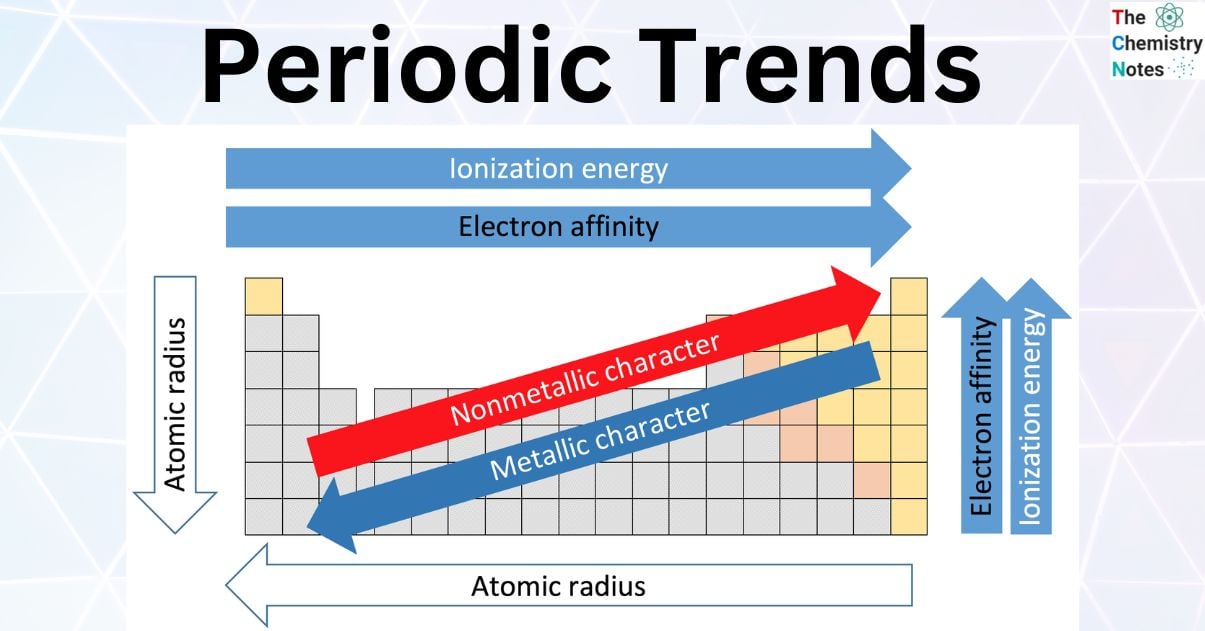
What is the trend for an increase in the following: atomic radius, ionization energy, and electronegativity?
Determine whether the following ions are paramagnetic or diamagnetic:
Cu+
Br+
Zn2+
Cu+ diamagnetic
Br+ paramagnetic
Zn2+ diamagnetic
Write the correct formula and charges for the following polyatomic ions:
Nitrate, sulfate, phosphate, hydroxide, acetate, carbonate, bicarbonate, ammonium, and cyanide.
NO3-, SO42-, PO43-, OH-, CH3COO-, CO32-, HCO3-, NH4+, CN-
Draw the most correct lewis structure for CSN-
Draw the correct lewis structure for H2Se and determine the following:
Both geometries, hybridization, bond angle, and polarity
Tetrahedral, bent, sp3, >109.5o, polar