If temperature and amount of gas are constant, doubling the pressure will increase volume
What is Boyle's Law?
The inner part of an atom that contains the proton and the neutron is called the __________.
The reason we calculate mass of products and reactants in a chemical equation
What is the Law of Conservation of Mass?
The atoms of one element replace the atoms of another in a compound during a chemical reaction.
What is Single-Replacement?
What is the white microscope cabinet?
If volume remains constant, pressure varies with temperature.
What is Gay-Lussac's Law?
These particles have a positive charge and are found inside the nucleus.
What are Protons?
Identify the product in this equation by name
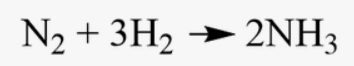
What are 2 molecules of Ammonia?
When two substances combine, and energy is released in the form of heat and light.
What is Combustion?
The tool used to measure any liquid during a lab
The combination of the 3 intial gas laws expressing the relationships between pressure, temperature, and volume at the same time.
What is The Combined Gas Law?
These particles are located within the nucleus, and have no charge.
What are Neutrons?
Identify the reactants in this equation by name
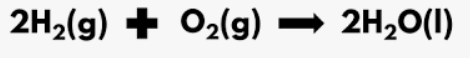
What are 2 molecules of Hydrogen gas and 1 molecule of Oxygen gas?
A single compound breaks down into two or more elements or new compounds after a chemical reaction
What is Decomposition?
The container used to collect strained or extracted liquid?
What is an Erlenmeyer Flask?
When pressure remains constant, volume increases/decreases according to temperature.
What is Charles's Law?
These particles are negatively charge and are found outside the nucleus.
What are Electrons?
A part of a chemical equation that limits the extent of the reaction
Where two or more reactants produce a single product
What is Synthesis?
The tool used to measure weight of a substance for a lab
What is a Balance?
A Law that describes the physical behavior of a theoretical gas in terms of pressure, volume, temperature, and number of moles.
What is the Ideal Gas Law?
Which of these is least stable using the octet rule?

What is Chlorine?
The product of the actual yield of a reaction and the theoretical yield that should have resulted?
What is percent yield?
Classify this reaction

What is Double-Replacement?
The tool used to transfer specific amounts of liquid to be tested on a well plate or in a test tube/beaker
What is a Pipette?
Who is most likely to be injured during a lab because they refuse to honor their teacher's request of full PPE (pants) during labs?
Who is Cody Minturn?