True or False:
Bananas are radioactive.
True!
A small fraction of the potassium in bananas naturally occurs as the radioactive potassium-40 isotope
This type of substance is made up of only one type of atom. It can not be separated into smaller, individual parts.
An Element
Physical or Chemical?
A nail rusting
Chemical
Atomic numbers of an atom are the same as the number of what in an atom?
![]()
Protons
Early people thought you could turn iron and lead into what other metal?
Gold
These particles have a positive charge and are found inside the nucleus.
Proton
When two substances are mixed together an CAN be separated by physical means.
(ex. evaporation, filtering, sorting)
Mixture
Physical or Chemical?
Charcoal/Wood in a fire turn to ash after a few hours.
Chemical
The up and down columns on a periodic table are called _______.

Groups
The earliest form of chemistry was called _______.
Alchemy
When atoms of two or more different kinds are combined and can only be separated by chemical reactions.
Compounds
Physical or Chemical?
Sugar dissolves in water.
Physical
Strontium has an atomic mass of _________.
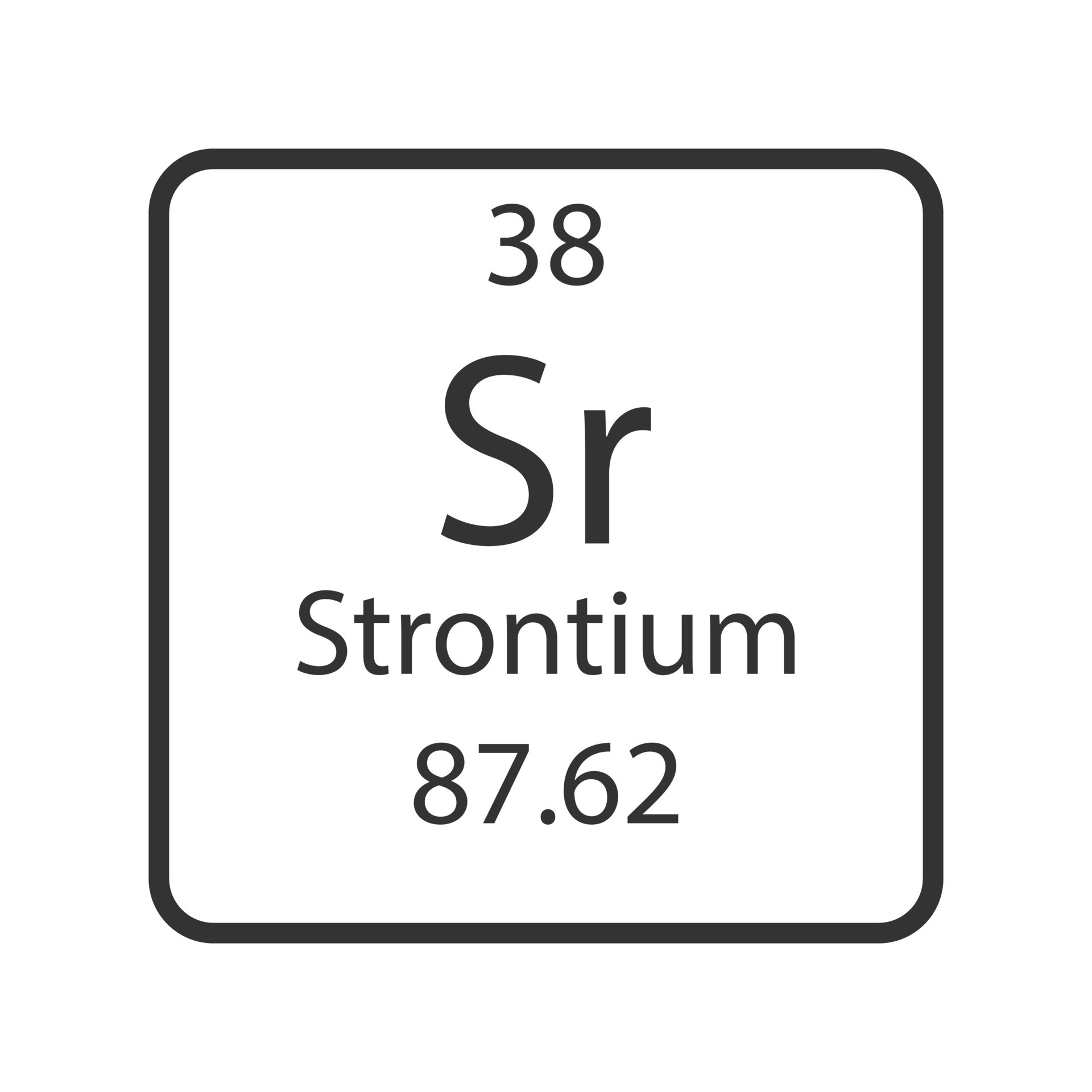
87.62
Names for elements comes from the English or __________ name.
Latin
These particles are negatively charge and are found outside the nucleus.
Electrons
Homogenous and Heterogeneous
During this type of change, the object doesn't change its identity. No new substance is formed.
Physical Change
(ex. torn paper, boiling water)
Why does hydrogen have an atomic number of 1?
because it only has one proton
People first realized that atoms had both protons and electrons in which decade?
(your guess should end in a zero, ex: the 1710s means anywhere in 1710-1719)
The 1910's
These electrons are farthest from the nucleus, they can be found in the last energy level.

Valence Electrons
This is an example of what type of mixture? (Image: Trail Mix)

Heterogeneous Mixture
(Why? We can separate physically)
What are three ways you can identify a chemical change has occurred?
- A new substance was formed
- A color change
- A gas produced
- An odor/smell is produced
- Heat, light, or sound produced
What two parts of an atom are added up to find the atomic mass of an element?
Protons and Neutrons
(the heaviest subatomic particles!)