Draw all possible substitution products for this reaction with pseudoephedrine: 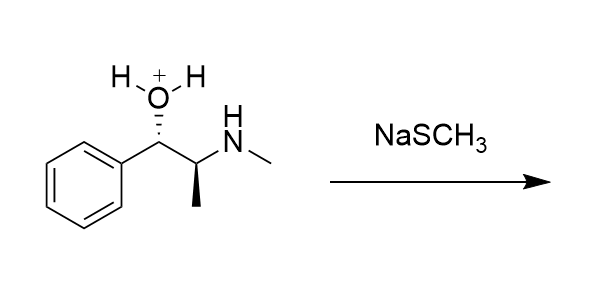
Only the right is possible through SN2...
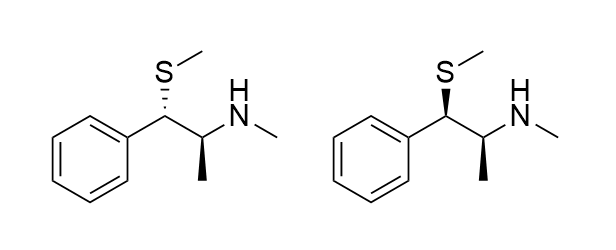
What mechanism(s) can lead to this possible product?
SN1 only: see retention of stereocenter
Which of these bases are considered too "bulky" to undergo substitution reactions?
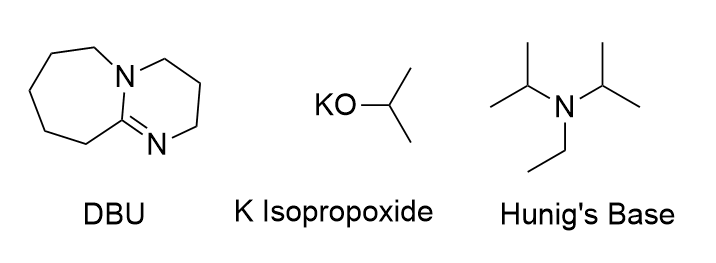
DBU and Hunig's Base
Draw all possible elimination products for this reaction with pseudoephedrine: 
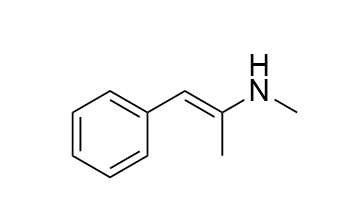
Cannot proceed through E2...
What predominant mechanism can lead to this product?
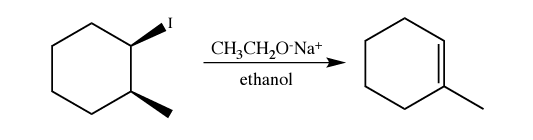
E2 (E1 will lead to same product but unlikely to occur)
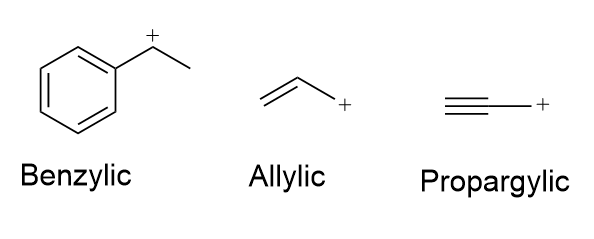
What molecular structures stabilize carbocations for unimolecular reaction mechanisms?
Predict the product and what mechanism:
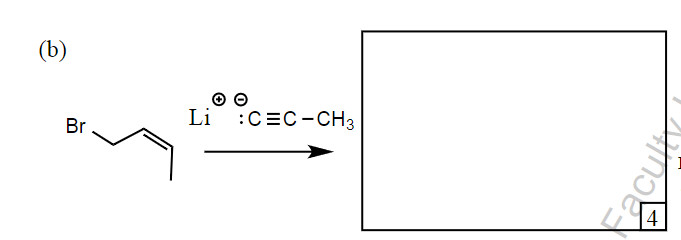
Undergoes SN2
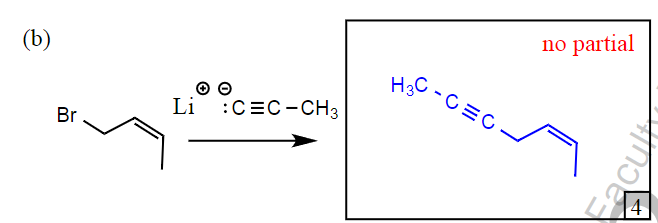
What mechanism does this reaction proceed by?
SN1
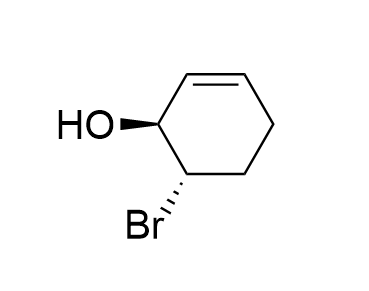
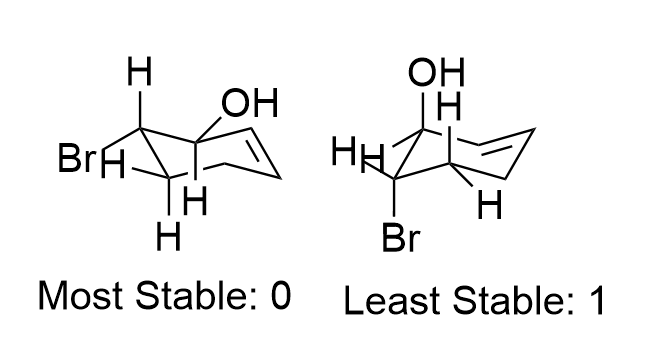
How many possible E2 products are there for each conformer of this substrate?
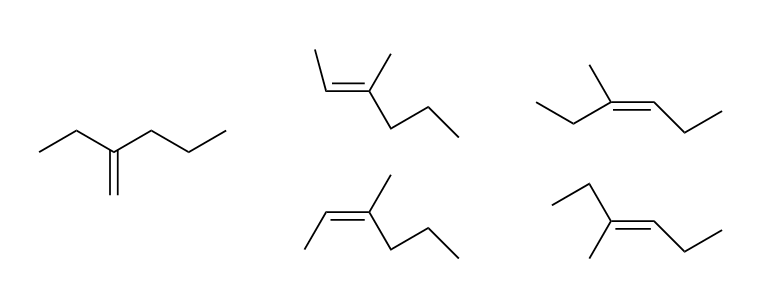
How many possible products are there for this E1 reaction?
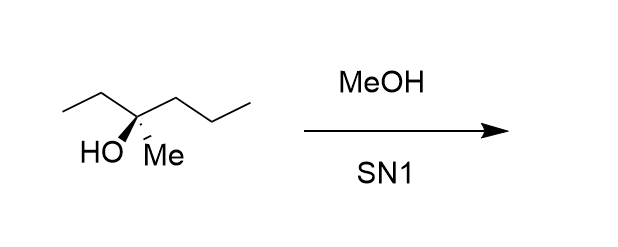
Five!
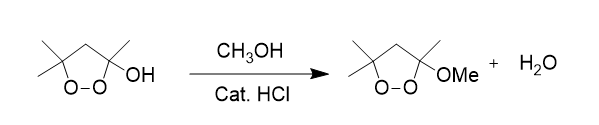
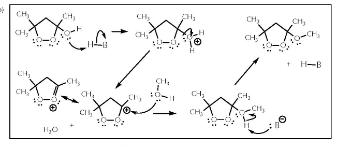
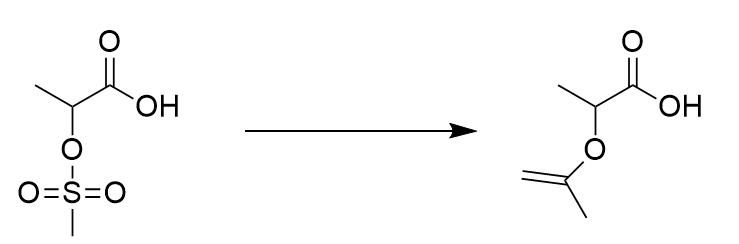
Predict both the product and the mechanism for this transformation of lactic acid:
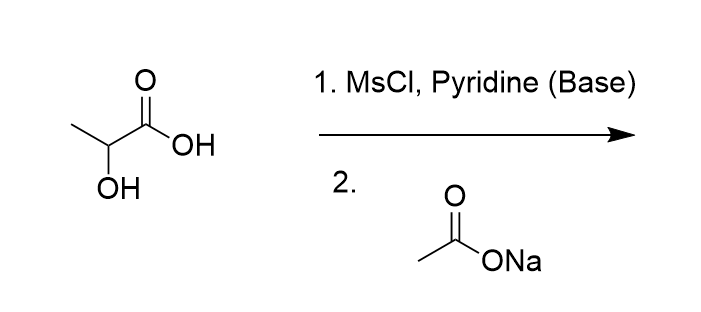
SN2
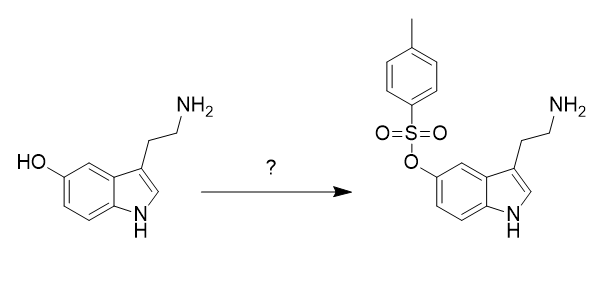
What are the conditions to protect the alcohol on the molecule below (serotonin)?
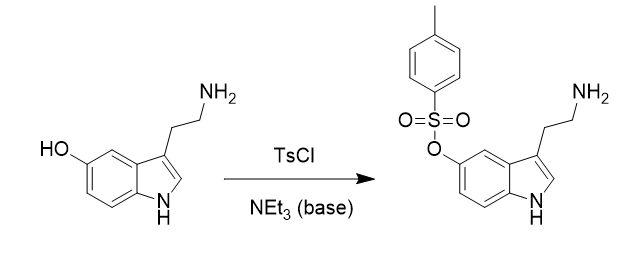
Predict all possible products for this reaction with correct stereochemistry:
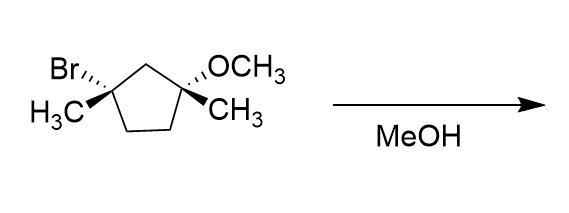
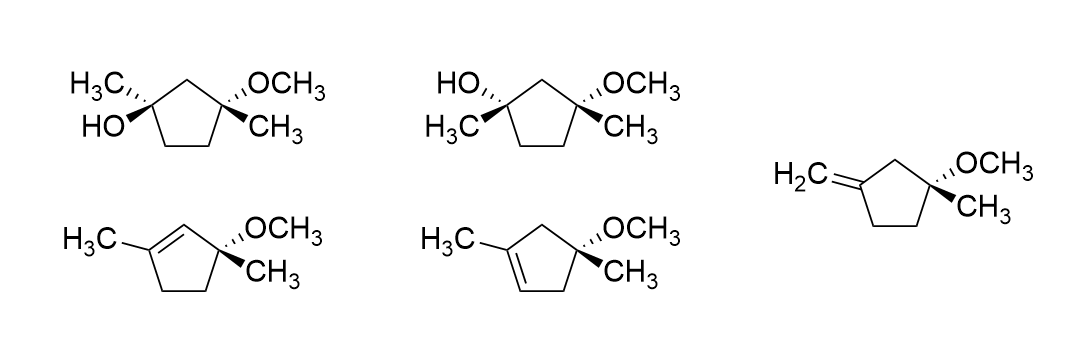
Which diastereomer of 1-bromo-4-t-butylcyclohexane, the cis or the trans, undergoes elimination more rapidly when treated with sodium ethoxide (NaOCH2CH3)?
