IF GIVEN THE MODEL BELOW. WHAT SUBATOMIC PARTICLE WOULD YOU USE TO IDENTIFY THE ELEMENT ON THE PERIODIC TABLE?
PROTONS
WHAT IS THE NET FORCE ACTING ON THE BOX BELOW?
400 N DOWN
Waxing light is on the ___________ and waning light is on the _____________.
Waxing is after the ______ moon and waning is after the _______ moon
growing, shrinking
right, left
new, full
Would the element be classified as a metal, nonmetal or metalloid?
Color - Yellow
Surface Appearance - Dull
Conducts Electricity and Heat - Poor
Can be stretched into wire - No
Reaction to being hit by a hammer - Shatters or breaks
Non-metal
What year will your class graduate high school?
2028
WHAT WOULD YOU LOOK AT ON THE PERIODIC TABLE TO FIGURE OUT WHAT ELEMENT HAS SIMILAR CHEMICAL PROPERTIES OR REACTIVITY TO BROMINE?
OTHER ELEMENTS IN GROUP 7
HOW MANY PHOSPHORUS ATOMS ARE IN THE CHEMICAL FORMULA BELOW?
Cu3(PO4)2
2
The Earth tilted toward the sun is the season __________. The earth tilted away from the sun is the season _____________.
Spring comes after the _________ season and fall comes after the ________ season.
summer, winter
winter, summer
Students were asked to find the identify of a wooden object. The mass of the wooden object is 33.0 grams and the volume is 42cm3. What is the density of the wooden object.
What type of wood is the object?
Bamboo 0.30- 0.40 g/cm3
English Elm 0.55 - 0.60 g/cm3
Plum 0.65 -0.80 g/cm3
Box 0.95 - 1.20 g/cm3
Density - 0.79 g/cm3
Plum Wood
When is the last day of school?
May 23rd
KRYPTON, Kr
A 89 kilogram runner produces a force of 84 Newtons as he runs. How fast is the runner accelerating? Round to the nearest hundredth.
0.94 m/s2
Which location is experience daytime in the summer?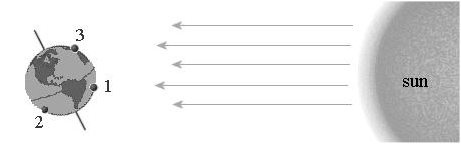
Point 1
At which position is the potential energy the greatest? At which position is the kinetic energy the greatest?
Potential - A
Kinetic - D
ROYGBIV
Red, Orange, Yellow, Green, Blue, Indigo, Violet
WHAT ELEMENT IS A NONMETAL WITH SIX VALENCE ELECTRONS IN PERIOD 3?
SULFUR (S)
Sodium sulfate (Na2SO4) is used in many products.
What elements are represented in the formula and how many of atoms of each element are present?
Sodium, Sulfur, Oxygen
2 atoms of sodium, 1 atom of sulfur, and 4 atoms of oxygen.
In this diagram would you use FENS or SNEF?
At what position(s) would we observe a full moon and a 1st quarter moon?
FENS
Full moon - Position 3
First quarter - position 1
Name one of the human body systems pictured below and write its function or job in the body.
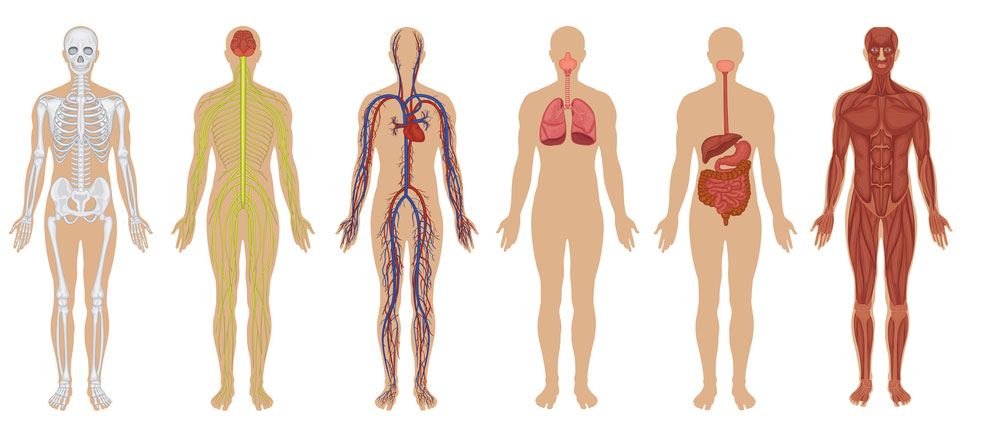
Skeletal - give the body structure and protects organs
Nervous - send signals to and from the brain
Circulatory - delivers blood and nutrients throughout the body
Respiratory - moves oxygen through the body
Digestive - breaks down food
Muscular - allows the body to move
When is the next leap year?
How many days are in February during a leap year?
2028
29 days
IS THE STATEMENT ABOUT ELECTRONS TRUE OR FALSE.
ELECTRONS ARE LOCATED OUTSIDE THE NUCLEUS, ARE SMALLER THAN NEUTRONS, WITH A POSITIVE CHARGE.
FALSE
ELECTRONS HAVE A NEGATIVE CHARGE
A chemical equation is shown below.
Ca(OH)2 + H2SO4 --> CaSO4 + 2H2O
74g ? 136 g 36g
According to the Law of Conservation of mass how many grams of sulfuric acid were used in the reaction?
98 g
Sept 1st - Waxing Gibbous
Sept 5th - Full Moon
Sept 9th - ?
Sept 12th - Third Quarter Moon
What moon phase would have been observed on Sept 9th?
Waning Gibbous
Is the cell below a plant or animal cell?
What is the role of the nucleus? What is the role of the mitochondria?
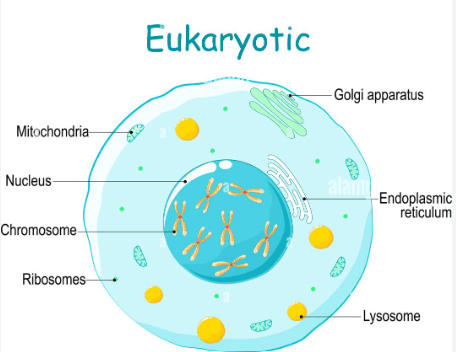
Animal Cell
Nucleus - brain or control center, holds genetic material
Mitochondria - power house of cell, produces energy
How many continents are there?
Name all the continents you can!
7 continents
Europe, Asia, Africa, South America, North America, Australia, Antartica