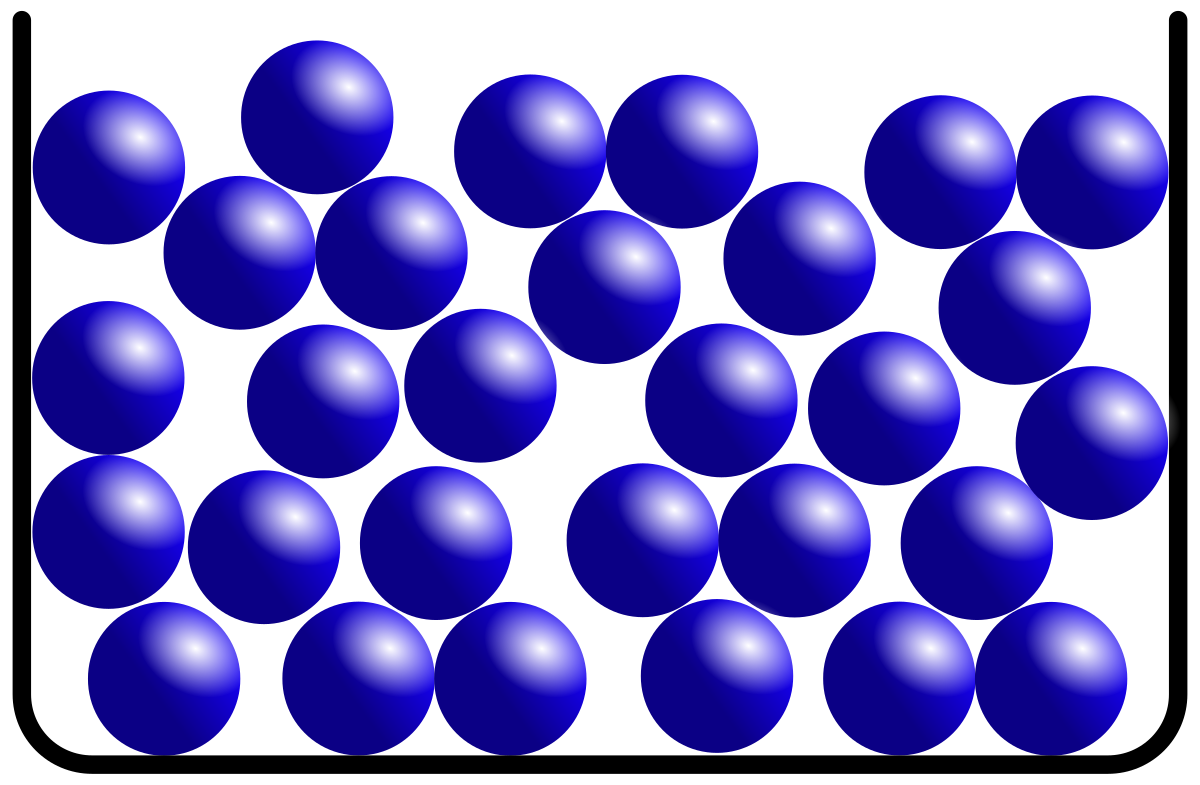
Particles have strong intermolecular forces in a fixed shape and volume with little free space between particles
What is the particle arrangement of a solid?

Neutral particles move freely past one another and don't stay connected
What is the molecular motion of a gas?
Particles are confined to a definite volume but no definite shape and have little free space between one another
What is the particle arrangement of a liquid?
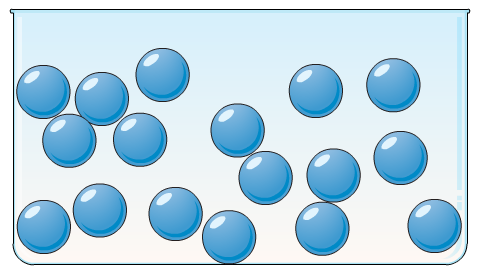
Particles move freely past one another, don't stay connected, and produce electricity due to extremely high thermal energy
What is the molecular motion of plasma?

Neutral particles are not limited to a fixed shape or volume and have weak intermolecular forces
What is the particle arrangement of gas?

Particles are locked in place and cannot move past each other
What is the molecular motion of a solid?
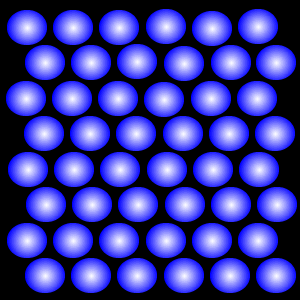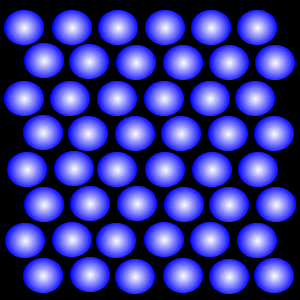
Charged particles are not limited to a fixed shape or volume and have lots of free space between one another
What is the particle arrangement of plasma?

Particles can flow past one another but stay in contact
What is the molecular motion of a liquid?
Name the two different states of matter being described: particles are not limited to a fixed shape or volume (Bonus: name the difference between the states)
What are gas and plasma? (Only plasma has charged particles)
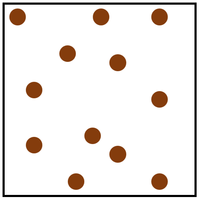
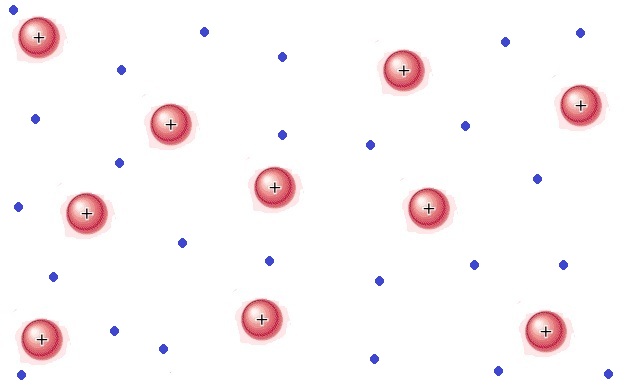
Name the two states of matter being compared: Both states have strong intermolecular forces, but only one has particles that can flow past one another
What are solid and liquid?