Phase Changes
Solids
Liquids
Gases
Matter
100
When water turns into ice.
What is freezing?
100
The movement of particles in the solid phase.
What is slow?
100
How the particles in the liquid phase move.
What is medium?
100
The way that particles move in this phase.
What is fast?
100
A tiny particle that makes up all matter.
What is an atom?
200
When a liquid turn into a gas.
What is evaporation?
200
The shape of these substances.
What is regular?
What is form?
what is irregular?
200
The shape of substances in the liquid phase.
What is irregular?
What is none?
200
What the volume of a gas depends on.
What is a container?
200
Two atoms that are joined together.
What is a molecule or compound?
300
A gas turning into a liquid.
What is condensation?
300
The amount of space between the molecules in the solid state.
What is a little?
What is close?
300
The volume of substances in the liquid phase is determined by this.
What is a container?
300
The chemical formula for steam.
What is H20?
300
A chemical substance that cannot be broken down or only one type of atom.
What is an element?
400
When liquid particles become gas particles they,
loses or gains energy.
Gain Energy
400
The chemical formula for the compound that makes up glaciers and the polar ice caps?
What is H20?
400
The chemical formula of the compound that makes up most of your body and covers most of the surface of the earth.
What is H2O?
400
A greenhouse gas that is also in the exhaled breath and used by plants.
What is CO2?
400
The theory that explains how atoms behave.
Particle Theory
500
In order for gas particles to become solid particles, particles need to lose or gain energy.
Lose energy
500
Materials that are usually solids and are good conductors of heat and electricity.
What are metals?
500
Review the heating curve:

What state of matter is described at point 3.
Liquid
500
What is this?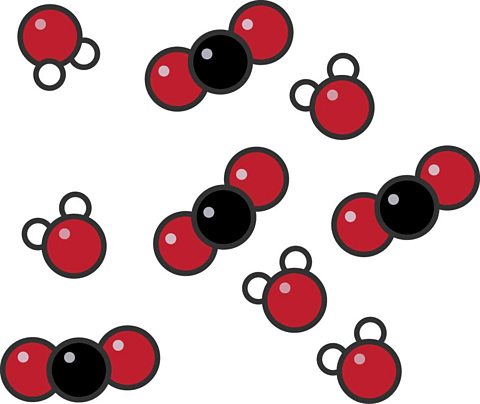
Mixture of compounds
500
The name of a molecule that is made up of more than one type of element and can be broken down during a chemical reaction.
What is a compound?