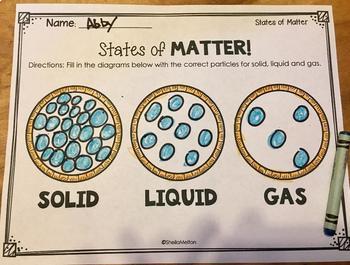
Name the 3 states of matter.
Solid, gas, and liquid
Physical change is a change in shape, size, texture, or state.
It does not create a new substance and can be reversible.
Define chemical change.
Chemical change is when 2 or more substances mix to create a new susbtance.
It cannot be reversed.
What is the process called for when a solid turns into a liquid?
Melting
Draw a model to show how molecules are pack for each state of matter.
Dissolving sugar/salt/baking soda in water
Ripping paper
Cutting food
Give two examples of a chemical change.
Baking soda and vinegar
Red phenol, baking soda, and calcium chloride
Nails rusting
Baking/Cooking food
What's the process called when a liquid turns into a solid?
Freezing
Give an example of a solid, gas, and liquid.
Solid - Ice
Liquid - Water
Gas - Steam
What does the levels of attraction tells us about molecules?
The higher the level of attraction, the more molecules will stick.
The lower the level of attraction, the less molecules will stick.
What is our rule about matter?
Matter cannot be created or destroyed.
What's the process called when a gas turns into a liquid?
Condensation
Name the state(s) of matter: A cup of water
Solid - Cup
Liquid - Water
What's the process called when a solid turns into a gas?

Sublimation
Name the state(s) of this matter: Bubbles!

Liquid on the outside
Gas on the inside
Explain why this model shows a physical change.
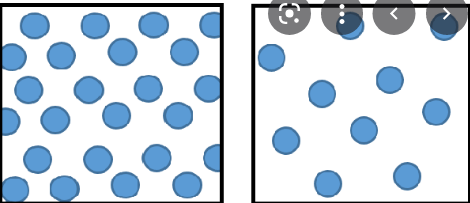
The model shows a physical change because the molecules are changing from a liquid to a gas. No new substance was created since only states were changed.
The molecuels are first packed loosely, then it gets packed very far apart.
Explain why iron rusting is a chemical change based on the molecules seen here at the nanoscale.
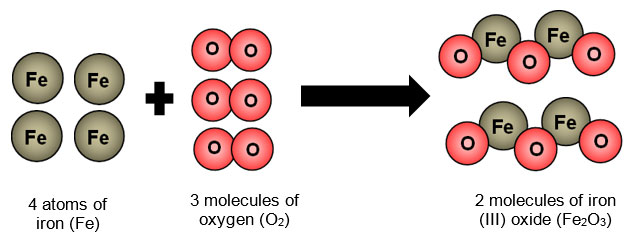
No matter was destroyed or created during this change.
What's the process called when a gas turns into a solid?

Deposition
