Anything that takes up space and has mass is called this.
What is matter?
This force is represented in "atm" (atmospheres) and increases temperature when it is increased.
What is pressure?
The phase change from a liquid to a solid is called this.
What is freezing?
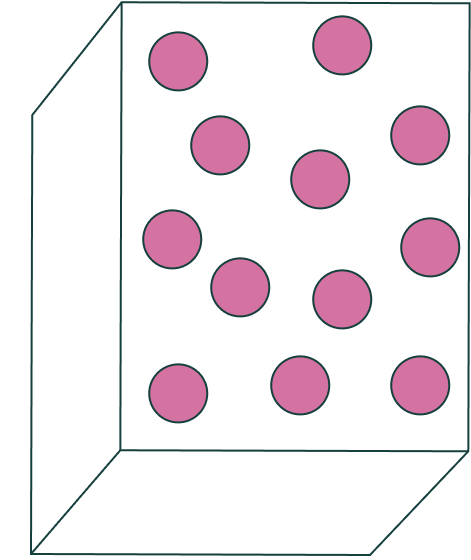
The state of matter of these molecules.
What is a gas?
The moment in which a substance or molecules exist in all three states.
What is the triple point?
A substance that can be deformed easily is said to have a high _____.
What is plasticity?
Any matter that has a definite shape and volume is called this.
What is a solid?
This type of energy is in motion.
What is kinetic energy?
The phase change from a liquid to a gas is called this.
What is boiling?
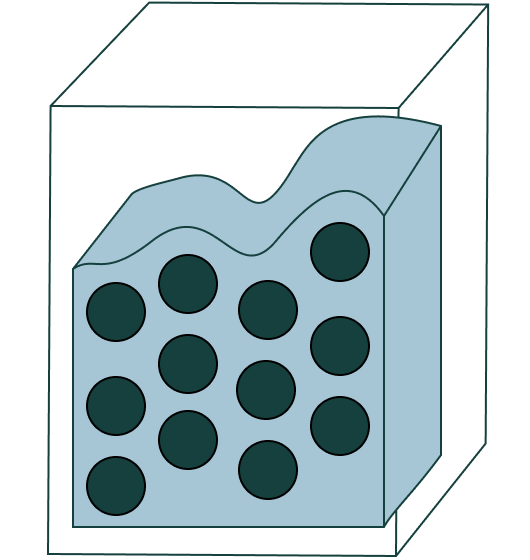
The state of matter of these molecules.
What is a liquid?
Any process that releases energy, or heat.
What is Exothermic?
These alloys have a high plasticity under normal heating conditions, but when heated up they revert back to their original shape.
What are Shape Memory Alloys?
This form of matter has a no definite shape but does have a definite volume.
What is a liquid?
This type of energy is considered at rest, or "loaded", ready to be used.
What is potential energy?
The phase change from a solid to a liquid is called this.
What is melting?
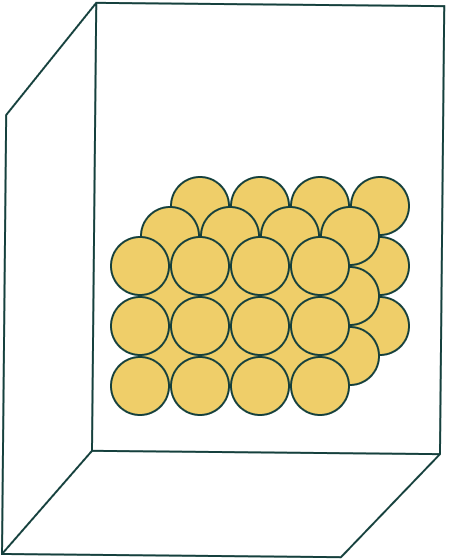
The state of matter of these molecules.
What is a solid?
The moment when a gas and liquid are considered indistinguishable.
What is the critical point?
Normal stress is any force applied perpendicular to a surface, while this stress is force applied parallel to the surface.
What is Shear Stress?
This matter has no definite shape and no definite volume.
What is a gas?
The measurement of thermal, kinetic energy of the individual particles within a substance.
What is temperature?
The phase change from a liquid to a gas is called this.
What is vaporization?
The phase change shown in this picture.

What is condensation?
Any process in which energy, or heat, is being added or absorbed.
What is Endothermic?
This is the word for a liquid's resistance to flow.
What is viscosity?
This matter is an ionized, electrically charged molecule in the supercritical state.
What is a plasma?
At this temperature, molecules stop vibrating or moving.
What is absolute zero? (0 degrees Kelvin, -273 degree Celsius)
This type of phase change strips electrons from molecules from intense heat.
What is ionization?
:max_bytes(150000):strip_icc()/sublimation-of-dry-ice-co2-solid-co2-changes-directly-from-solid-to-gas-128108785-5768263e5f9b58346ad1d386.jpg)
The phase change shown in this photos of dry ice (solid CO2)
What is sublimation?
The changing of a gas directly to a solid.
What is deposition?
This law states when you increase the pressure of a gas, the volume decreases; and if you decrease the pressure of a gas, the volume of that gas increases.
What is Boyle's Law?