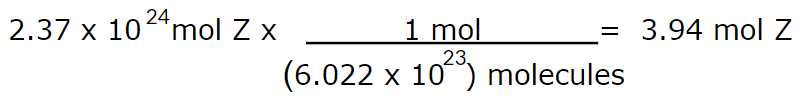Where do numbers for a mole to mole ratio come from?
Where does mass or grams come from?
Coefficients (big numbers in a balanced reaction)
Periodic table
2 K + Cl2 -> 2 KCl
If a student performs an experiment using 2.5 grams of chlorine, how many moles of potassium chloride will be formed?
0.07 mol KCl
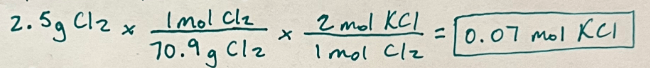
Is the following reaction balanced or unbalanced?
FeO3 + Br2 --> Fe3Br2 + O2
Unbalanced
What is the molar mass of Mg2F
67.72 grams
how many steps is mol to mol question?
1 step
4 Fe + 3 O2 ->2 Fe2O3
If you have 1.33 moles of iron(Fe), how many grams of oxygen(O2) would be required?
31.92 g O2
What does the law of conservation of mass state?
Mass cannot be created nor destroyed (mass of reactants must equal mass of products)
Convert 100 g of TiO to moles.
1.57 moles
How many steps is a grams to grams question?
3
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
If you have 25 moles of K2SO4 how many grams of H2SO4 will be produced?
2452.25 g H2SO4
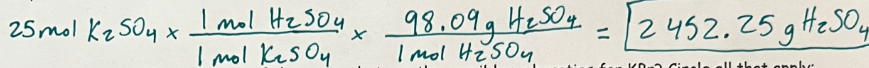
Balance the following reaction:
______ Rb2Se--> ____ Rb2 + ____Se2
Convert 3.0 mol of H2O to grams
54.06 g
The substance you want to get rid of goes on the _________________ of the fraction
BOTTOM
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
If you have 5.4 moles of K2SO4 how many moles of KBr will be produced?
10.8 mol KBr
How many atoms are in a mole?
How many molecules of water do you have if you have 2.5 moles of water?
1.51 x 1024 molecules H2O
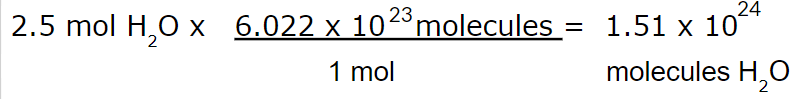
4 Fe + 3 O2 ->2 Fe2O3
If you have 7.5 mols of iron, how many mols of oxygen would be required?
5.63 mol O2
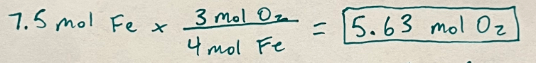
K2SO4 + 2 HBr -> H2SO4 + 2 KBr
If you have 15 g of HBr, how many grams of KBr will be produced?
22.06 g KBr
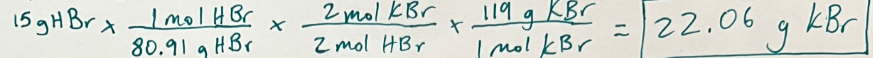
What is Avogadro's number used for?
(Hint: 3 answers)
Atoms, molecules, particles
How many moles of compound Z is 2.37 x 1024 molecules of Z?
3.94 mol Z