Process that allows for measuring the substances going into a reaction and products coming out of a reaction.
What is Stoichiometry?
This is Avogadro's number.
6.02214 x 1023?
Aluminum (Al) will react with sulfuric acid (H2SO4) in the following reaction.
2 Al(s) + 3 H2SO4(l) → Al2(SO4)3(aq) + 3 H2(g)
This is how many moles of H₂SO4 will react with 18 mol Al.
What is 27 mole H2SO4?
Hydrogen peroxide breaks down, releasing oxygen, in the following reaction:
2 H2O2(aq) → 2 H2O(1) + O2(g)
This is the mass of water produced when 5 mole of O2 are produced from this reaction.
What is 180 grams H2O?
This element has an electron configuration of 1s22s22p63s23p3?
What is Phosphorus (P)?
The number of grams of a substance per one mole.
What is Molar Mass?
If you have one mole of a substance, you have this much of the substance.
What is 6.022 x 1023?
This is how many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?
2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g)
What is 8 mole Na?
Hydrogen peroxide breaks down, releasing oxygen, in the following reaction:
2 H2O2(aq) → 2 H2O(1) + O2(g)
This is the mass of oxygen produced when 1.840 mol of H2O decomposes.
What is 29.44 grams of O2?
This periodic table family is composed of a set of chemically unreactive elements with due to their already full valence shells.
What is Noble Gases?
Quantitative analysis of substances involved in a chemical reaction.
What is Stoichiometry?
What is 17 g/mol?
Phosphorus burns in air to produce a phosphorus oxide in the following reaction:
4 P(s) + 5 O2(g) → P4010(s)
This is the mass in grams of phosphorus that is needed to produce 3.25 mol of P4010.
What is 403 grams P?
Sodium carbonate reacts with nitric acid according to the following equation.
Na2CO3(s) + 2 HNO3 → 2 NaNO3 + CO2 + H2O
This is how many moles of Na2CO3 are required to produce 100.0 g of NaNO3.
What is 0.5882 mole Na2CO3?
Chemical bond in which two atoms of opposite net electrical charges attract and stick together.
What is Ionic Bond?
Conversion factor that relates moles of any two substances.
What is Molar Ratio?
This is the molar mass of Sulfur Hexafluoride (rounded).
What is 146 g/mol?
I2 is produced by the reaction of 0.4235 mol of CuCl2 according to the following equation:
2 CuCl2 + 4 KI → 2 CuI+ 4 KCl + I2.
This is how many grams of I2 is produced.
What is 53.8 grams I2?
Chlorine gas can be produced in the laboratory by adding concentrated hydrochloric acid to manganese(IV) oxide in the following reaction:
MnO2 + 4 HCl → MnCl2 + 2 H2O + Cl2
This is the mass of MnO2 needed to produce 25.0 g of Cl2.
What is 30.7 grams MnO2?
Chemical bond in which electrons are delocalized and flow through a common pool of cations of the same element.
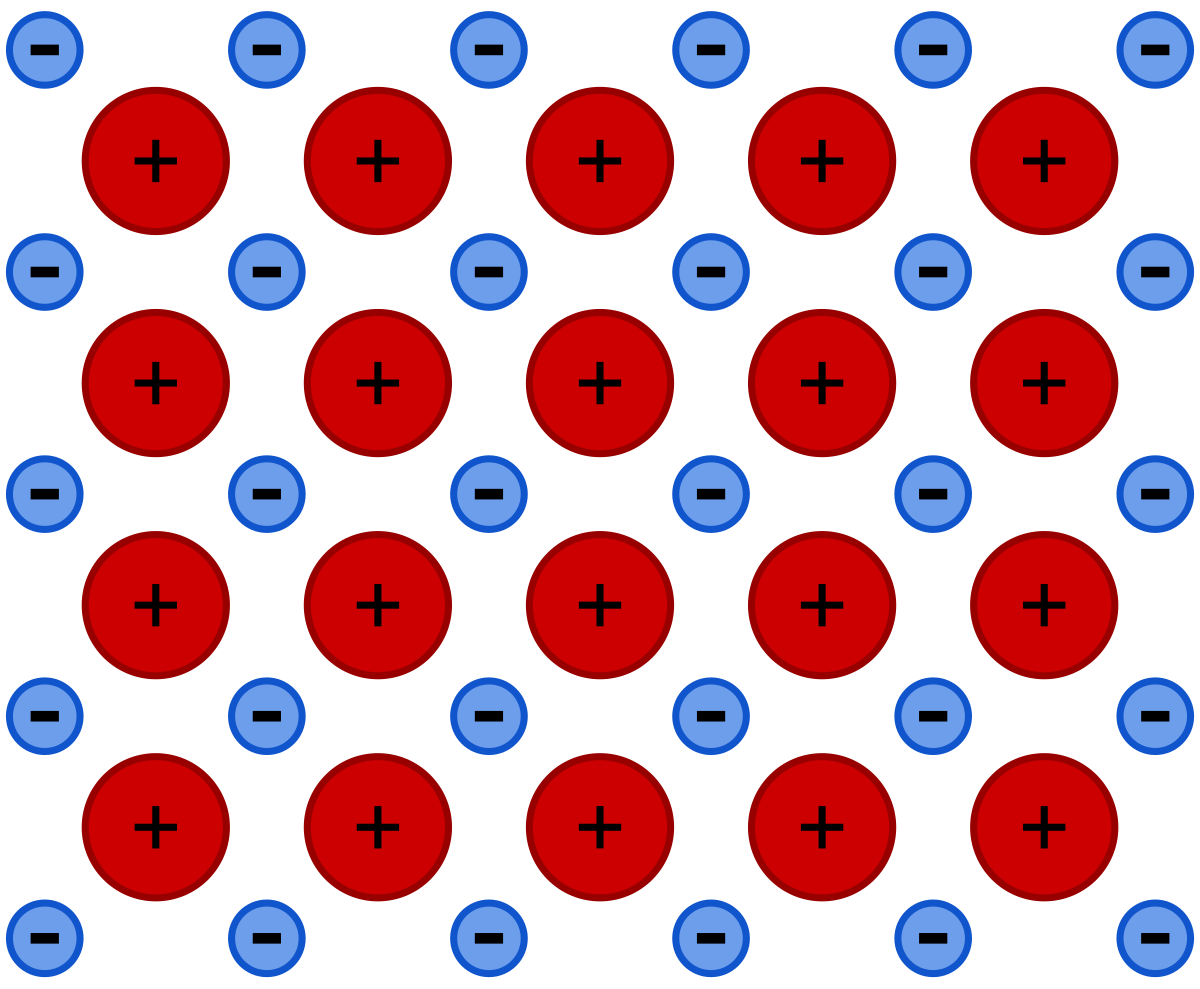
What is Metallic bond?
Not necessarily a definition, but...
This scientist laid the groundwork for discovering that if you have 6.022 x 1023 of a substance, you'll have exactly as many grams as its atomic mass of one atom.
What is Amadeo Avogadro?
This is the molar mass of H2SO4 (sulfuric acid)? (round your answer)
What is 98 g/mole?
Carborundum is silicon carbide, SiC, a very hard material used as an abrasive on sandpaper. It is prepared by the reaction of pure sand, SiO2, with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. This how much SiO2 is required to produce 3.00 g of SiC.
What is 4.50 SiO2?
This is the mass of silver bromide produced from 22.5 g of silver nitrate in the following reaction:
2 AgNO3(aq) + MgBr2(aq)→ 2 AgBr(s) + Mg(NO3)2(aq)
What is 24.9 grams AgBr?
This is the chemical formula for Iron(III) Sulfide.
What is Fe2S3?