The molar mass of CO2.
What is 44.01
The number of moles of magnesium in 3.05 x 1022 atoms.
What is .05 moles
The percent by mass of oxygen in CO2 (molar mass = 44.01)
What is 72.71%
The empirical formula for P4O10
What is P2O5
 The number of moles of CO2 produced when 5.00 moles of Fe2O3 reacts.
The number of moles of CO2 produced when 5.00 moles of Fe2O3 reacts.
What is 15.00 moles CO2
This could be the actual formula for NH4
What is N2H8, N3H12, N4H16, N5H20
This much Fe was ACTUALLY produced.
What is 3.34 g
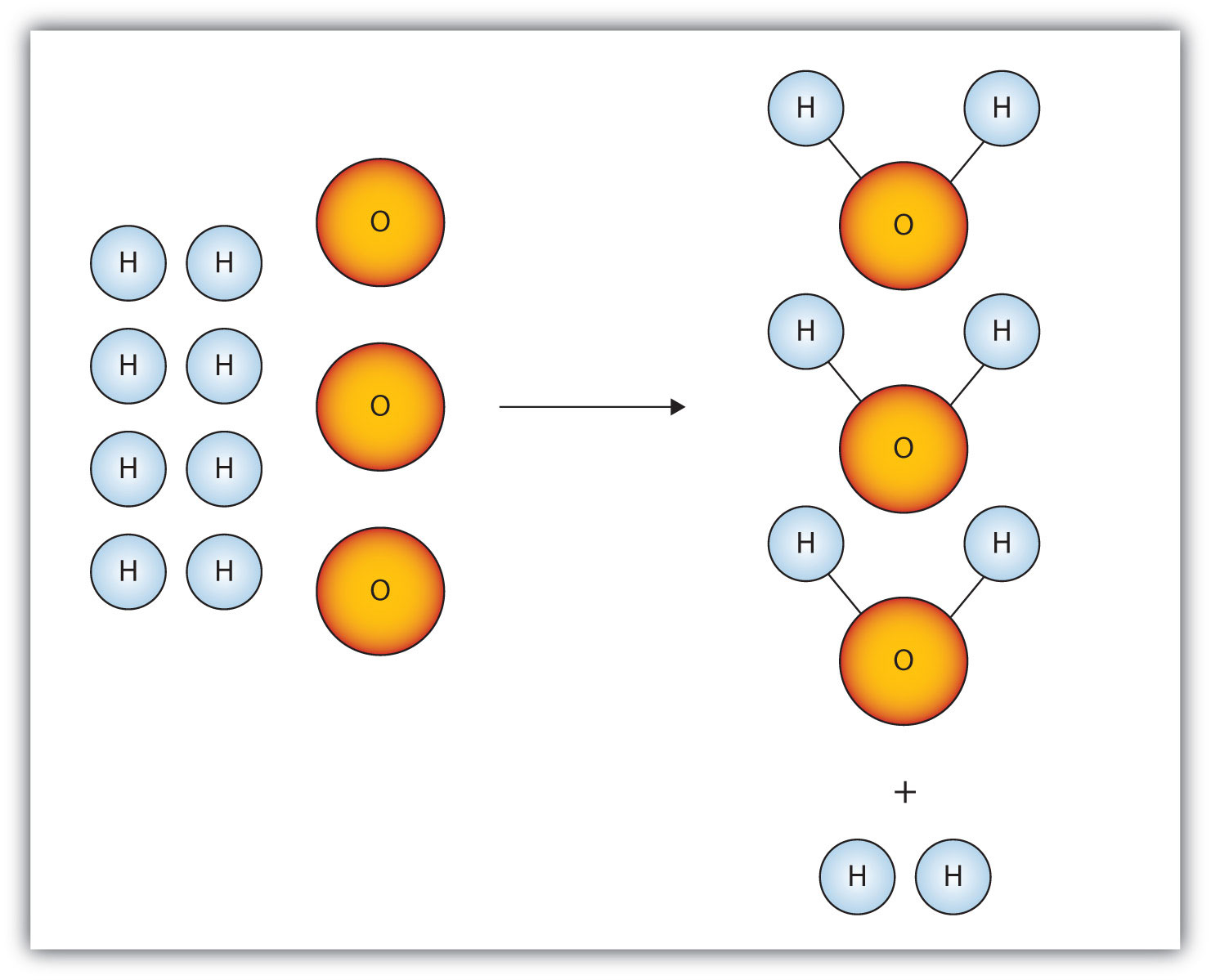
The limiting reactant here
What is oxygen
The molar mass of Li3P.
What is 51.80
The number of molecules in 4.05 moles of glucose, C6H12O6.
What is 2.44 x 1024
The percent by mass of lithium in LiF (molar mass = 25.94).
What is 26.75%
The Empirical formula for Cr3Cl9
What is CrCl3
 The number of molecules of Fe produced from 248 grams of CO (Molar Mass = 28.01).
The number of molecules of Fe produced from 248 grams of CO (Molar Mass = 28.01).
What is 3.55 x 1024
This could be the actual formula for SO2
What is S2O4, S3O6, S4O8, S5O10
This much Co was ACTUALLY produced.
What is 2.91 g

The limiting reactant here.
What is nothing
The molar mass of Fe2S3
What is 207.88
The number of moles in 1.21 x 1025 atoms of phosphorus.
What is 20.10 moles
The percent by mass of hydrogen in C3H4 (molar mass = 40.062).
What is 10.06%
The empirical formula for Fe2(SO3)2
What is FeSO3
 The number of grams of Fe2O3 (Molar Mass = 159.7) required to react with 6.23 moles of CO.
The number of grams of Fe2O3 (Molar Mass = 159.7) required to react with 6.23 moles of CO.
What is 331.64 grams
This could be the actual formula for P2O3
What is P4O6, P6O9, P8O12, P10O15
This is the percent yield of Co in this experiment.
What is 55.17%

The limiting reactant here
What is hydrogen
The molar mass of Na3PO4
What is 163.94
The number of moles in 11.3 L of argon gas at STP.
What is .50 moles
The percent by mass of copper in CuCl2 (molar mass = 134.45).
What is 47.27%
The empirical formula for C8H10O2N4
What is C4H5ON2
 The number of moles of Fe produced from 4.93 x 1024 molecules of CO.
The number of moles of Fe produced from 4.93 x 1024 molecules of CO.
What is 5.46 moles
This could be the actual formula for CH2O
C2H4O2, C3H6O3, C4H8O4, C5H10O5
This is the percent yield of Na in this experiment.
What is 78.58%
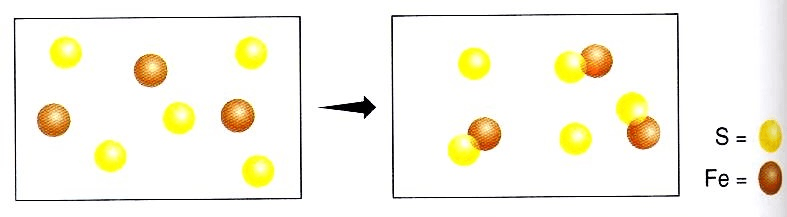 The limiting reactant here.
The limiting reactant here.
What is Fe
The molar mass of Mg3(PO4)2
What is 262.84
The number of grams in 2.6 mol of lithium bromide (molar mass = 86.84).
What is 225.78 grams
The percent by mass of calcium in CaO (Molar Mass = 56.08).
What is 71.47%
What is the empirical formula for C15H25O10
What is C3H5O2
The number of grams of CO2 (Molar Mass = 44.01) in 7.38 x 1024 molecules.
What is 539.52 grams
This could be the actual formula for C3H4O5
What is C6H8O10, C9H12O15, C12H16O20
This is the ACTUAL mass of Mg that was produced AND the percent yield of Mg in this experiment.
What is 6.19 g and 85.50%
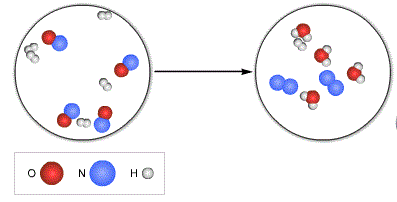
The limiting reactant here.
What is oxygen