1N2 + 3H2 --> 2NH3 What is the mole ratio of N2 and H2?
1mol of N to 3mol of H
How many grams are in 1.3 moles of CH4?
20.8 grams
How many moles are present in 34 grams of Cu(OH)2?
0.35 mol Cu(OH)2
What is the molar mass of O2?
32 g/mol
What is a limiting reactant?
the reactant that runs out first
What is the mole ratio of methane to water?
CH4 + 2O2 --> CO2 + 2H2O
1 mol CH4 to 2 mol H2O
The mass of one mole of a substance. The sum of all the atomic masses in grams. Is called?
Molar Mass
A conversion factor that relates the amounts in moles of any two substances involved in a chemical reaction.
mole ratio
Mg + H2O --> Mg(OH)2 + H2
Mg + 2H2O --> Mg(OH)2 + H2
What is the limiting reactant? 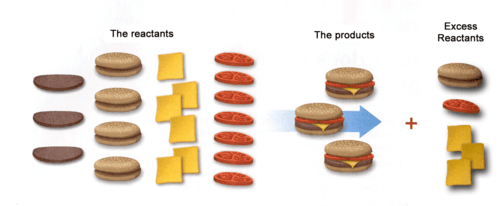
The Meat
How many moles of water can be formed from 30 moles of methane? 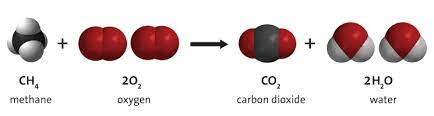
60 mol H2O
Sodium chloride is produced from its elements through a synthesis reaction. What mass of the Sodium reactant would be required to produce 25.0 mol of sodium chloride?
574.75g Na
States that mass is neither created nor destroyed during ordinary chemical or physical reactions. (This is why we balance chemical equations!)
the law of conservation of mass
Mg + 2HCl --> MgCl2 + H2 What mass of hydrogen is produced from the reaction of 75 g HCl (MM=36.5 g/mol) and excess Mg?
2.0 g H2
N2 + 3H2 --> 2NH3 In the reaction above, a student reacted 28 g of nitrogen with an excess of hydrogen. According to the students measurements, the reaction produced 30 g of product. The theoretical yield was 34 g. What is the student's percent yield?
88%
Ammonia, NH3, is widely used as a fertilizer and in many household cleaners. How many moles of ammonia are produced when 6 mol of hydrogen gas react with an excess of nitrogen gas?
4 mol NH3
This is the molar mass of Copper(II) Sulfate.
160 g/mol
This is how many moles of LiOH are required to react 20 moles of CO2 in the following reaction: CO2 + 2LiOH --> Li2CO3 + H2O
40 moles
Sodium and chlorine react to produce sodium chloride (table salt; MM=58.5 g/mol). Determine the amount of product that can be produced from 24.7 g of sodium and excess chlorine.
62 g NaCl
The double-replacement reaction between silver(I) nitrate and sodium bromide produces silver bromide, a component of photographic film.
What is the balanced equation?
Ag(NO)3 + NaBr → AgBr + Na(NO)3
Hydrogen and oxygen gases react under a specific set of conditions to produce water. How many moles of oxygen would be required to produce 5.0 moles of water?
2.5 mol O2
This is the percent yield of CuS given that you start with ? 15.5 g Na2S and ? 12.1 g CuSO4. The actual amount of CuS produced was ?3.05g . Reaction: Na2S + CuSO4 → Na2SO4 + CuS
42.1%
This questions is worth 1000 points! SURPRISE! Chlorine gas reacts with potassium bromide to produce potassium chloride and bromine gas. How many grams of potassium chloride can be produced from 300. g each of chlorine and potassium bromide?
188 g KCl
When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed. If 15 g CuCl2 (MM=134.5 g/mol) react with 20 g NaNO3 (MM=85 g/mol), how much sodium chloride (MM = 58.5 g/mol) can be formed?
13.0 g NaCl
Fe2O3(s) + CO(g) → Fe(s) + CO2(g)
What is this balanced equation?
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)